FDA approves MelaFind, a device that helps dermatologists decide whether to order a skin biopsy of suspected melanoma.
FDA approves MelaFind, a device designed by Mela Sciences, that helps dermatologists decide whether to order a skin biopsy of suspected melanoma- the deadliest form of skin cancer which is responsible for 75% of skin cancer fatalities.
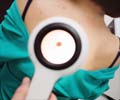
The device uses a special dermoscope to view the skin through a
thin layer of alcohol or oil. The device takes 'digital images of skin growths' and compares them to a database of thousands of scans for signs of melanoma. MelaFind provides information about pigmented skin lesions to help determine whether to order biopsy.
MelaFind cannot be used to confirm a clinical diagnosis. It is intended to aid, but not replace, melanoma screening by expert dermatologists. This technology is a breakthrough in early melanoma detection.
Source-Medindia