The U.S. Food and Drug Administration (FDA) has allowed marketing of the Eclipse System for the treatment of fecal incontinence (FI) in adult women.
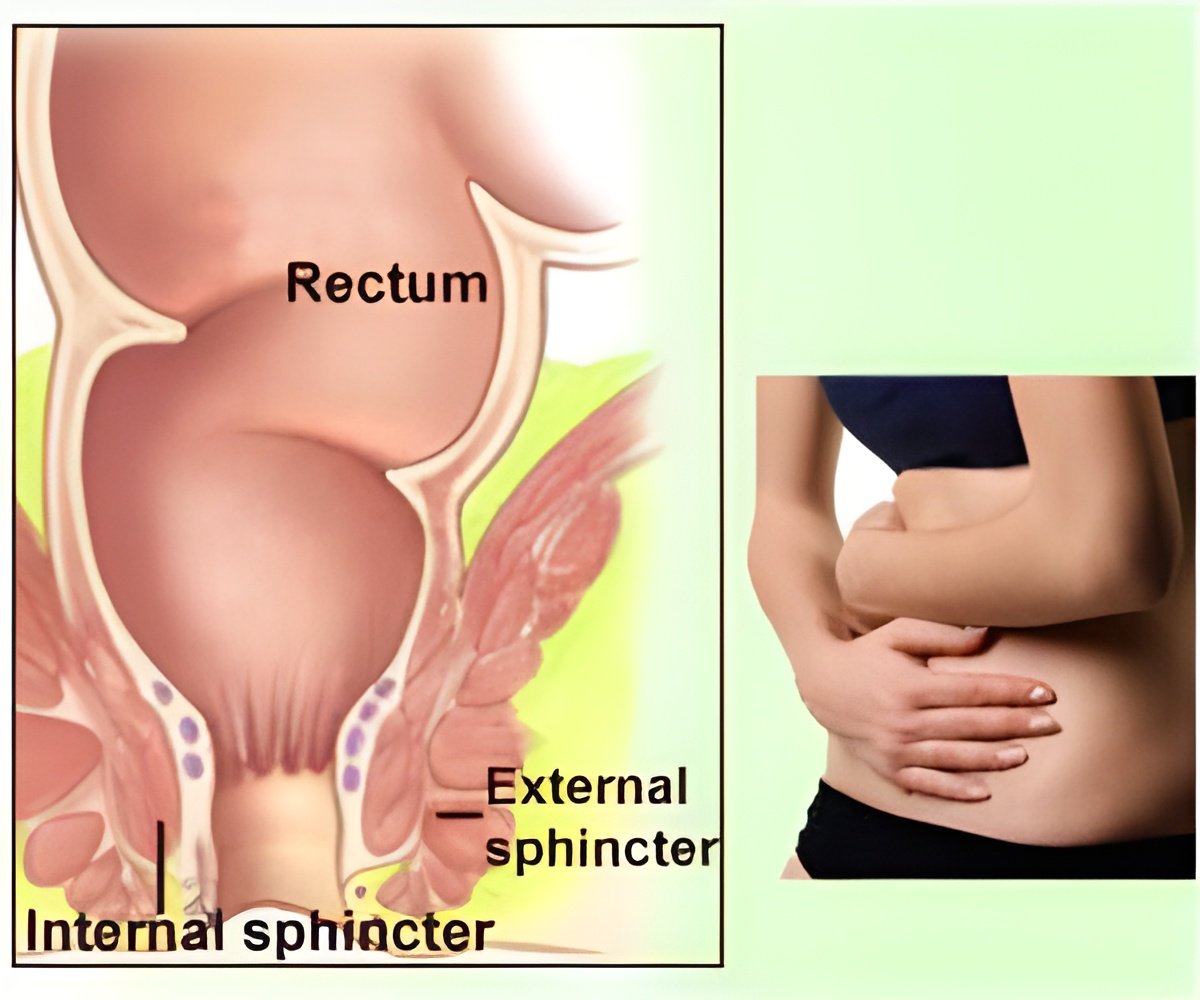
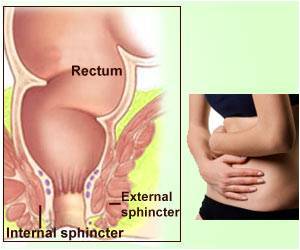
The most common cause of FI is damage to the muscles around the anus. Vaginal childbirth can damage the anal sphincters or their nerves, which is why fecal incontinence impacts women about twice as often as men.
William Maisel, M.D., M.P.H., deputy director for science and chief scientist in the FDA’s Center for Devices and Radiological Health said that, “Current treatment options for fecal incontinence include drugs, dietary changes, exercise, and surgery. The Eclipse System provides an additional treatment option for women who suffer from this condition.”
The Eclipse System is intended to treat FI in women 18 to 75 years old. The device includes an inflatable balloon, which is placed in the vagina. Upon inflation, the balloon exerts pressure through the vaginal wall onto the rectal area, thereby reducing the number of FI episodes.
The device is initially fitted and inflated by a clinician (with the use of a pump) and after proper fitting, the patient can inflate and deflate the device at home as needed. The device should be removed periodically for cleaning.
The Eclipse System is manufactured by Pelvalon, Inc., in Sunnyvale, California.
Source-Medindia