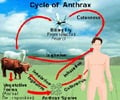
GlaxoSmithKline’s injectable drug. raxibacumab, has been approved by the US FDA for treating inhalable anthrax.

Raxibacumab has been developed by GSK’s Human Genome Sciences and is a man-made protein that works by mimicking the naturally produced antibodies and blocks the toxins produced by the anthrax bacteria
Source-Medindia