Medical authorities in the United States have approved the use of the drug Avastin to help combat the most common form of kidney cancer, the Swiss pharmaceutical giant Roche announced Monday.
Medical authorities in the United States have approved the use of the drug Avastin to help combat the most common form of kidney cancer, the Swiss pharmaceutical giant Roche announced Monday.
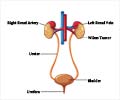
Avastin has been given the seal of approval by the Food and Drug Administration (FDA) to be used to treat metastatic renal cell carcinoma in combination with the drug Interferon Alpha, Roche said in a statement.According to the results of the third phase of a clinical study, patients who used a combination of the two drugs lived nearly twice as long without their disease getting worse compared to those who only used Interferon Alpha.
"This underscores our belief in the important clinical benefits that Avastin delivers as we push forward with our ongoing research programmes in more than 30 tumour types," said William Burns, chief executive of Roche's pharmaceuticals division.
Kidney cancer is the eighth most common type of cancer in the US, accounting for some 13,000 people last year.
Avastin has been available since the end of 2007 in Europe as a first line of treatment against advanced kidney cancer.
The news comes after the European Commission last week approved the use of Avastin as a treatment against breast cancer.
Advertisement
LIN