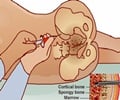
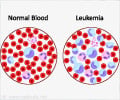
A company that makes products for extracting stem cells from a variety of sources, SynGen Inc., of Sacramento, CA, has received the FDA nod for its SynGenX-1000 platform.

Philip Coelho, CEO of SynGen, said “This ‘next generation’ SynGenX™-1000 System improves the recovery of stem and progenitor cells from cord blood units, which should increase the number of clinical grade units available for transplantation. The CryoPRO-2™ simplifies the cryopreservation workflow for cord blood bank personnel and provides a medical record of the process. The SynGen™ DataTrak software captures the data associated with processing each cord blood unit for the blood bank’s records.”
Source-Medindia