FDA has approved lumasiran subcutaneous injection to treat adults and children with primary hyperoxaluria type 1 (PH1)- an ultra- rare genetic disorder.
‘FDA has approved lumasiran subcutaneous injection to treat adults and children with primary hyperoxaluria type 1 (PH1)- an ultra- rare genetic disorder.The most common side effects of Lumisiran was reaction at the site of injection and abdominal pain.’
Read More..




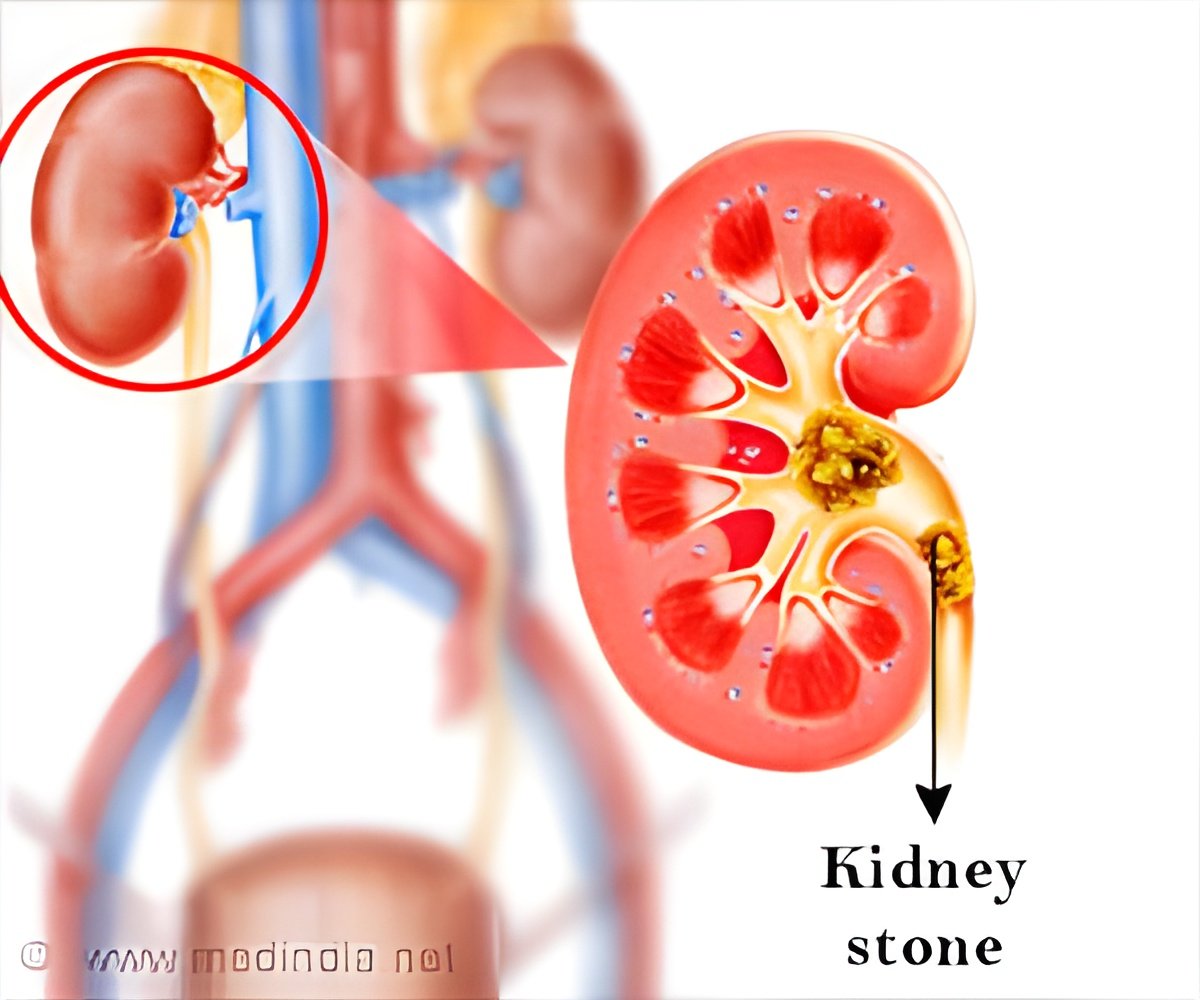
Patients with PH 1 produce very high levels of oxalate. These oxalates have a tendency to combine with calcium to form kidney stones. This can lead to progressive kidney damage and eventually kidney failure. Such patients may need dialysis (a process that purifies blood outside the body). If the kidney’s condition deteriorates oxalate levels can increase and accumulate in other organs including bones, heart, eyes etc. Read More..
“The approval of oxlumo (lumasiran) represents a great triumph of community involvement to address a rare disease. It is a result of input from patients, treating physicians, experts and sponsors at a patient-focused drug development meeting and through other collaborative efforts,” said Norman Stockbridge, M.D., Ph.D., director of the Division of Cardiology and Nephrology in the FDA’s Center for Drug Evaluation and Research.
Lumasiran decreases oxalate production. It was evaluated in two studies in patients with PH1 – randomized, placebo- controlled trial in patients six years and older and an open label study in patients younger than six years of age. For the studies the patients ranged in age from 4 months to 61 years at the first dose.
Studies done on patients older than 6 years
In the first study the 26 patients received a monthly injection of Lumasiran followed by a maintenance dose every three months, 13 patients received placebo (no drug – inactive injection) .
Advertisement
Studies on patients younger than 6 years of age
Advertisement
The most common side effects of Lumasiran were reaction at the site of injection and abdominal pain.
Lumasiran for PH 1 is considered as a breakthrough therapy. It has been allotted the orphan drug status designation, which provides incentives to assist and encourage drug development for rare diseases.
In addition, the manufacturer received a rare pediatric disease priority review voucher. The FDA’s rare pediatric disease priority review voucher program is intended to encourage development of new drugs and biologics to prevent and treat rare diseases in children
Source-Medindia