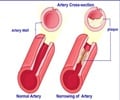
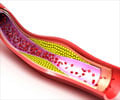
Sanofi confirmed that it has been asked by the USFDA to assess the potential neurocognitive side effects of its experimental cholesterol drug.
However the FDA said it was “aware of concerns raised with neurocognitive adverse events” and has asked the two companies to assess the side effects.
Amgen Inc is also developing a similar drug, evolocumab, and the company revealed that it has also been contacted by the agency. “Similar to other companies developing PCSK9 inhibitors, Amgen has been in communication with the FDA, and we will continue to investigate the potential for cognitive impairment in our program”, the company said in a statement.
Source-Medindia