BioDirection’s Tbit System, the new traumatic brain injury device has been awarded breakthrough device designation from The FDA.
‘The Tbit System has the potential to deliver actionable information to the physician where minutes matter. Longer term, our technology has the potential to support a full continuum of care ranging from stratification of injury, prognosis and return to play and activity.
’





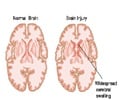
“The Tbit System is designed to measure the body’s response to trauma and provide a rapid point-of-care test result in less than two minutes from a single drop of blood, while current technology may run three to four hours or more and require serum testing in a central laboratory,” said James Wylie, BioDirection executive chairman. Because the device is portable, it could be used in the emergency department at a hospital, with potential for future use at the point-of-injury. The ability to diagnose brain injuries at an earlier stage could facilitate more appropriate treatment decisions, while also minimizing unnecessary head CT scans.
“Blunt trauma injuries that impact the head and brain require the rapid identification of comorbidities to rule out or confirm the potential of intracranial haemorrhage that may require some form of surgical intervention,” said Brian McGlynn and BioDirection founder and chief technical office.
The company is currently working on rapid point-of-care products (POCT) for the objective diagnosis and management of concussion and other TBI. Around one million people in the UK visit A&E departments due to TBI each year, highlighting the significant cost savings that could be achieved by the adoption of these technologies.
Source-Newswise