SAPIEN XT Transcatheter Heart Valves can be used for patients with aortic stenosis who cannot undergo open-heart surgery.
‘FDA expands SAPIEN XT Transcatheter Heart Valve (THV) by making the device available to an expanded group of patients who have inoperable aortic valve stenosis.’





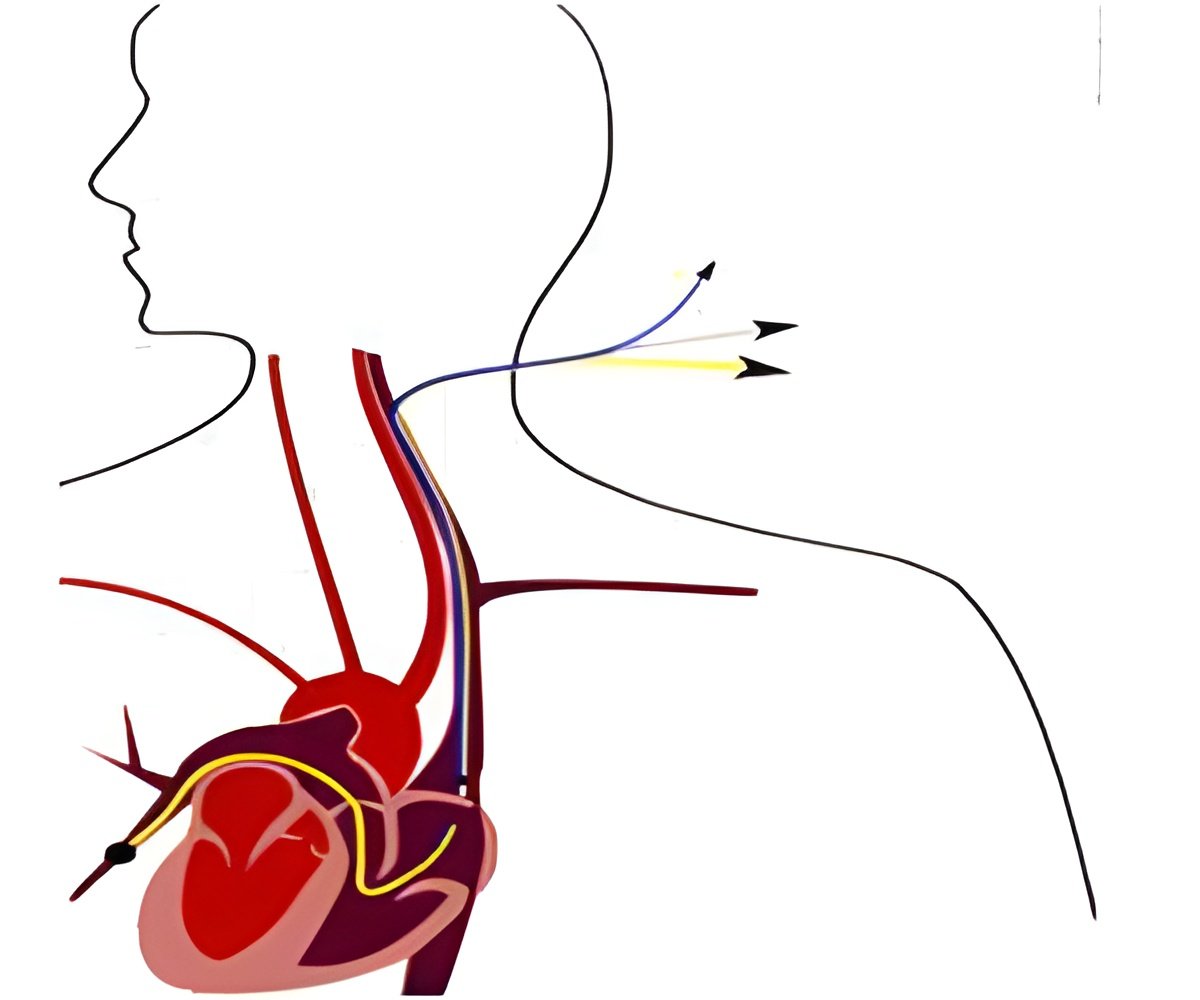
The catheter does not require open heart surgery or a heart machine. The device is compressed into a thin, flexible delivery catheter, inserted into an access point in the body and threaded to the site of the diseased valve.Edwards Lifesciences SAPIEN XT transcatheter heart valve for pulmonic valve replacement procedures was first approved by the FDA in 2012 with certain conditions. The catheter can be inserted through the femoral artery, the leg or the lower tip of the heart. These were the standard access points that were previously approved.
Now, the FDA has expanded the device to be inserted into the body at any other alternate points if the standards access points are inoperable in patients. For this change, the company has submitted data from the Transcatheter Valve Therapy Registry (TVTR) in the United States and THV device registries in Europe, along with data from FDA-approved clinical studies and peer-reviewed medical journals.
“Just two years after the THV entered the market for a specific patient population, data from the TVTR was used to support FDA approval that expands patient access to a life-saving therapy. Medical device registries like the TVTR, not only play an important role in the FDA’s post market surveillance system, they also collect robust and timely data that can be used to identify additional patient populations that benefit from the therapy,“ said Dr. Jeffrey Shuren, director of the FDA’s Center for Devices and Radiological Health.
“Leveraging clinical research inside the framework of a device registry to expand access to therapy for more patients is a new paradigm for the FDA, researchers, registry sponsors and the medical device industry. We believe this approach can be used with future well-designed device registries to speed patient access to important, well-evaluated therapies,” said Shuren.
Advertisement