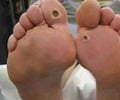
The US Food and Drug Administration revealed it will constitute a panel of independent experts to review the results of a new analysis.

However a GSK commissioned reanalysis of a study, called RECORD, by researchers at Duke University found no ‘statistically significant difference’ in heart safety when comparing older diabetes drugs to Avandia.
According to GSK spokeswoman Mary Anne Rhyne, the FDA panel will be meeting on June 5-6, speculating that the company may be asked to update the safety information that it had provided in 2010. “We haven't asked for any changes in the drug label or in distribution for Avandia”, Rhyne said.
Source-Medindia