The US Food and Drug Administration advisory panel will consider a new type of transcatheter heart value for treating patients with severe aortic stenosis.

The review follows a major clinical trial released earlier this year that experts hailed as a breakthrough for patients with severe aortic stenosis, offering them a viable alternative to risky open heart surgery.
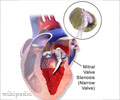
The condition is characterized by a clogged valve that makes the heart work harder to pump oxygen-rich blood. It affects nine percent of Americans over 65. Without treatment, up to half of patients die within two years.
The technique of inserting the bioprosthetic valve through a tube in the artery is less invasive than conventional surgery and showed similar survival rates, but also raised the risk of stroke and other major heart complications.
The research was part of the multi-year PARTNER study, the world's first randomized trial comparing the two methods, and was showcased at the American College of Cardiology conference in New Orleans in April.
The method lowered costs involved with rehospitalization in frail, elderly patients and was found to increase life expectancy by as much as 1.9 years.
Advertisement
"This probably will be seen as one of the biggest steps in cardiovascular medicine, as far as intervention is concerned, potentially in our lifetime," said David Moliterno, professor of medicine at the University of Kentucky, during the New Orleans meeting. Moliterno was not involved in the study.
Advertisement
Source-AFP