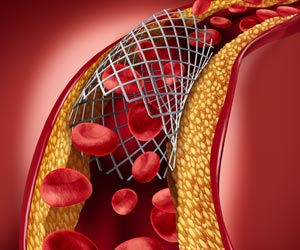
The FDA has revealed that it is working closely with stent manufacturers in order to find the exact cause that makes the newer class of heart stents to shrink

While the Ion stent has been approved by the FDA to be marketed in the United States, Boston Scientific has submitted an application to market Promus stents as well.
In a statement made to the Reuters news agency, the FDA said, “FDA is actively working with (drug-eluting stent) manufacturers, including Boston Scientific, to better understand longitudinal stent deformation with respect to its causes, predisposing underlying anatomic conditions, operator techniques that can reduce the likelihood of its occurrence, and treatment strategies should it occur.”
Source-Medindia