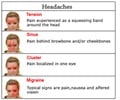
The government had warned the world's largest maker of generic drugs and 19 other companies that they are illegally selling migraine medicines without federal approval.
The government had warned the world's largest maker of generic drugs and 19 other companies that they are illegally selling migraine medicines without federal approval.
The Food and Drug Administration said the 20 warning letters were part of an effort to halt the marketing of unapproved and potentially dangerous drugs. The goal is to get the unapproved drugs off the market, agency officials said. The manufacturers could seek agency approval for the products.The prescription migraine treatments contain a drug called ergotamine tartrate. Ergotamine is derived from a rye fungus called ergot, from which a separate drug, the illegal hallucinogen LSD, also can be synthesized. Ergotamine is a vasoconstricting drug, meaning it narrows the blood vessels when taken.
The letters — dated Monday but not publicly disclosed until Thursday — went to companies that include a U.S. subsidiary of Israel's Teva Pharmaceutical Industries Ltd., the world's largest generics manufacturer. Other recipients included Iceland's Actavis and Sandoz Inc., a pioneer in the marketing of ergotamine tartrate that is now part of Switzerland's Novartis AG.
Messages left with all three companies were not immediately returned Thursday.
The FDA said the companies have 60 days to stop making the drugs and 180 days to stop distributing them. Otherwise, the companies are subject to seizure or injunction, the FDA said.
"Because these drugs don't have approval, we don't know how they were formulated or manufactured. We don't know if they are safe or effective," Deborah Autor, director of the office of compliance within the FDA's Center for Drug Evaluation and Research, told reporters.
Advertisement
There are five ergotamine tartrate drugs on the market that have FDA approval, including one made by Sandoz. The labels of those approved medications carry "black-box" warnings, the most severe the government can require, about the risk of drug interactions.
Advertisement
Source-Bio-Bio Technology
SRM