A high-profile HIV vaccine trial that was shown to work in just under a third of patients may have been hindered by feuding antibodies, says US researchers.
When its results were first published in 2009, experts hailed its 31 percent protection rate as a pioneering achievement, even though it fell short of hopes by providing only a partial shield against HIV.
A vaccine would have to offer 50 percent protection in order to be offered to the public.
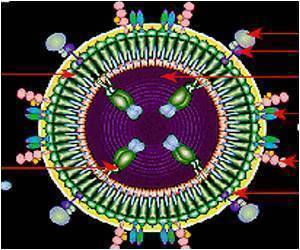
Experts are continuing to study the data for clues on what went wrong, and now they believe that an antibody that was induced by the vaccine, immunoglobulin A, was fighting against another that would have blocked the virus.
"We learned that a specific vaccine-induced immunoglobulin A can weaken the protective effect of immunoglobulin G," said Georgia Tomaras, director of the Laboratory of Immune Responses and Virology at Duke Human Vaccine Institute.
"IgA competes with IgG to bind to the same site on the virus's outer envelope that is exposed on infected cells," said Tomaras, lead author of a study in the Proceedings of the National Academy of Sciences that describes the research.
Advertisement
Tomaras said the latest findings offer clues toward how to induce more effective responses in future vaccines.
Advertisement
According to the World Health Organization, 34 million people worldwide were living with HIV at the end of 2011.
Source-AFP