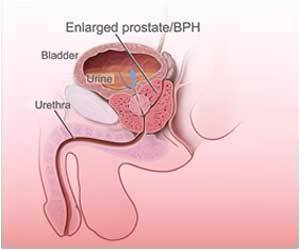
People who take Finasteride for male hair loss assume it to be safe, but there is insufficient information to make that judgment.
Lead author Steven Belknap said, "People who take or prescribe the drug assume it’s safe, but there is insufficient information to make that judgment. Of the 5,704 men in the Northwestern Medicine clinical data repository who were treated for male pattern baldness with finasteride, only 31% met inclusion criteria for the pivotal trials referenced in the manufacturer’s Full Prescribing Information. Thus, the available information from clinical trials does not apply to most of these men in Northwestern’s study population who took finasteride for male pattern baldness. For example, some men with hair loss who are taking finasteride have diabetes mellitus, high blood pressure or are taking other drugs such as diuretics or antidepressants that also increase the risk of sexual dysfunction. Duration of drug safety evaluation was limited to one year or less for 26 of 34 trials, but 33% of men in the Northwestern clinical data repository took finasteride for more than one year."
The study has been published in JAMA Dermatology.
Source-Medindia