What is India’s milestone development in cervical cancer prevention? A new indigenously developed vaccine for cervical cancer is going to be available at an affordable price.
Cervical Cancer Prevalence In India
India accounts for about a fifth of the global burden of cervical cancer, with 1.23 lakh cases and around 67,000 deaths per year. Almost all cervical cancer cases are linked to certain strains of human papillomavirus (HPV), a common virus that is transmitted through sexual contact.‘Cervavac, the first cervical cancer vaccine made in India to be priced between Rs.200 – 400 demonstrates an effective immune response against the human papillomavirus.’





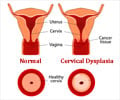
While the body’s immune system usually gets rid of the HPV infection naturally within two years, in a small percentage of people the virus can linger over time and turn some normal cells into abnormal cells and then cancer, according to the Centres for Disease Control and Prevention (CDC).Screening and vaccination are two powerful tools that are available for preventing cervical cancer. Still, there is little awareness among women about the prevention of this cancer and less than 10% of Indian women get screened.
All women aged 30-49 must get screened for cervical cancer even if they have no symptoms and get their adolescent daughters vaccinated with the HPV vaccine.
Developing New cervical cancer vaccine in India
Cervavac was developed by the Pune-based Serum Institute of India in coordination with the Government of India’s Department of Biotechnology (DBT). The project to develop the vaccine was implemented by the then secretary of the DBT, Dr. M K Bhan in 2011.Since then, 30 meetings of scientific advisory groups and site visits conducted by DBT have helped review the scientific merit of the entire journey to develop the vaccine. As per indications from the Serum Institute of India, the cost would approximate between Rs 200 to 400.
Cervavac received market authorization approval from the Drug Controller General of India on July 12 2022. HPV vaccines are given in two doses and data has shown that the antibodies that develop after both are administered can last up to six or seven years.
Advertisement
The biggest task will be in allocating adequate resources and manpower for vaccinating the massive demographic of adolescent girls aged between 9 and 15, to ensure that they are protected from HPV early on.
Advertisement
Source-Medindia