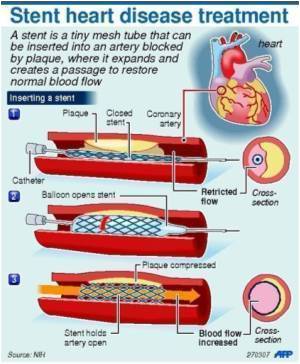
The stent has shown to be easily positioned across different lesions because of its high flexibility and maintains strength over considerable time periods

The stent has shown to be easily positioned across different lesions because of its high flexibility and maintains strength over considerable time periods.
FDA approval was based on submission of one-year data from the Occlusive/Stenotic Peripheral Artery Revascularization Study (OSPREY). The study findings indicate a low potential for stent fracture, simplified delivery system and 88.6% freedom from target lesion revascularization.
Dr. John Fritz Angle, Principal Investigator for the U.S. clinical trial said, "The MISAGO stent has a flexible design and good radial force that we found performed well in the superficial femoral artery. Peripheral artery disease can have devastating consequences but we believe the MISAGO stent offers a durable treatment option for superficial femoral artery disease."
Source-Medindia