A French maker of breast implants now feared to be at risk of rupture was warned as early as in 2000 by the US Food and Drug Administration of 'serious' quality control violations in saline implants.
The letter, seen by AFP, outlines a list of quality assurance problems the FDA warned "may be symptomatic of serious underlying problems in your firm's manufacturing and quality assurance systems."
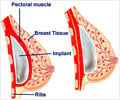
The company is currently under scrutiny not for its saline implants, but for its silicone implants. PIP is suspected of fraudulently using a poor-quality gel for those devices which is linked to leakage and inflammation problems.
France's health ministry has advised 30,000 women in France with PIP silicone breast implants to have them removed, saying that while there is no proven cancer risk, the prostheses could rupture.
Prosecutors in Marseille, near PIP's home base of La-Seyne-sur-Mer, have received more than 2,000 complaints from Frenchwomen who received the silicone implants, and have opened a criminal investigation into the firm.
Documents obtained by AFP showed tens of thousands of women in more than 65 countries, mainly in South America and western Europe, received silicone implants produced by PIP.
Advertisement
"Our warning letter was publicly available in 2000. Given the timeframe, I haven't been able to confirm whether any information was shared with France," said the spokeswoman, Erica Jefferson.
Advertisement
Source-AFP