A gene that scientific dogma insists is inactive in adults actually plays a vital role in preventing the underlying cause of most heart attacks and strokes.
‘The gene, Oct4, plays a key role in the development of all living organisms, but until now, scientists have thought it was permanently inactivated after embryonic development.’





"Finding a way to augment the expression of this gene in adult cells may have profound implications for promoting health and possibly reversing some of the detrimental effects with aging," said researcher Gary K. Owens, PhD, director of UVA's Robert M. Berne Cardiovascular Research Center. Unexpected Protective EffectThe gene, Oct4, plays a key role in the development of all living organisms, but scientists have, until now, thought it was permanently inactivated after embryonic development. Some controversial studies have suggested it might have another function later in life, but the UVA researchers are the first to provide conclusive evidence of that: Owens and his colleagues have determined the gene plays a critical protective role during the formation of atherosclerotic plaques inside blood vessels. The rupturing of these plaques is the underlying cause of many heart attacks and strokes.
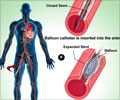
The researchers found that Oct4 controls the movement of smooth muscle cells into protective fibrous "caps" inside the plaques - caps that make the plaques less likely to rupture. The researchers also have provided evidence that the gene promotes many changes in gene expression that are beneficial in stabilizing the plaques. This is exciting, because studies suggest that it may be possible to develop drugs or other therapeutic agents that target the Oct4 pathway as a means to reduce the incidence of heart attacks or stroke. "Our findings have major implications regarding possible novel therapeutic approaches for promoting stabilization of atherosclerotic plaques," said Olga A. Cherepanova, PhD, a senior research scientist in Owens' lab.
One surprising finding from UVA's research: When the researchers blocked the effect of Oct4 in mice, they thought the atherosclerotic plaques might become smaller, because of the reduced number of smooth muscle cells inside. Instead, the plaques grew larger, less stable and more dangerous, stuffed with lipids, dead cells and other damaging components.
Advancing Regenerative Medicine While UVA's research has focused on how Oct4 offers cardiovascular protection, Owens and his colleagues believe the gene could also prove critical to the field of regenerative medicine, which investigates the growth and replacement of tissues and organs. The researchers believe that Oct4 and its family of target genes are activated in other somatic cells - the non-reproductive cells in the body - and play a key role in the cells' ability to repair damage and heal wounds. Studies to test this are under way in Owens' lab.
Advertisement
The UVA researchers suspect that at least some of the detrimental effects of aging, including the increased possibility of a plaque rupture, stem from a decrease in the body's ability to reactivate Oct4. "Finding a way to reactivate this pathway may have profound implications for health and aging," Owens said. "We think this is just the tip of the iceberg for controlling plasticity of somatic cells, and this could impact many human diseases and the field of regenerative medicine. Who knows, this may end up being the 'fountain-of-youth gene,' a way to revitalize old and worn-out cells. Only time will tell."
Advertisement