Scientists report a new advance in gene therapy for a rare, inherited form of blindness.

"Our concern was that the first treatment might cause a vaccine-like immune response that could prime the individual's immune system to react against a repeat exposure," said lead author Jean Bennett, professor of ophthalmology at the University of Pennsylvania.
Despite the risks, three adult patients from the initial study -- whose results were published in 2009 showing that some level of vision had been restored in all of them and six were no longer legally blind -- agreed to try the therapy in their untreated eye.
The trio were all better able to see in dim light afterward, and two were able to navigate obstacles in low-light situations. There were no reports of ill side-effects.
The study appears in the journal Science Translational Medicine.
"Patients have told us how their lives have changed since receiving gene therapy," said Bennett. "They are able to walk around at night, go shopping for groceries and recognize people's faces -- all things they couldn't do before."
Advertisement
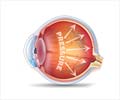
Caused by flaws in any one of around 13 key genes, LCA triggers severe loss of vision and abnormal eye movements in early infancy, usually leading to total blindness in the twenties or thirties.
Advertisement
The modified virus was injected into the eyeball, and infected the diseased cells -- in effect, acting like a Trojan horse to deliver the right DNA to the retina.
Twelve patients aged eight to 44 years were recruited in the small, experimental study, and were given the treatment in the eye that had the worst vision.
None of the patients recovered normal sight, but all of them had an at-least 100-fold increase in so-called pupillary light response, meaning the constriction of the pupil when exposed to light.
Six of the 12 recovered sufficient sight that meant they may no longer be classified as legally blind.
The best results were among four children aged eight, nine, 10 and 11.
Their first study was published by The Lancet in 2009.
Gene medicine is one of the most alluring areas of biotechnology, offering the theoretical promise of blocking or reversing inherited disease.
But this new frontier has also been hit by occasional setbacks, notably an unexpected or uncontrollable response from the immune system.
The pioneering treatment for LCA, though, has so far had no side effects and its benefits have remained unchanged years later, say the researchers.
More research is needed before the therapy can be confirmed safe, but the latest findings bode well for trying it in more of the initial subjects, particularly the youngest ones whose retinas have not degenerated as much as those of the adults.
Source-AFP