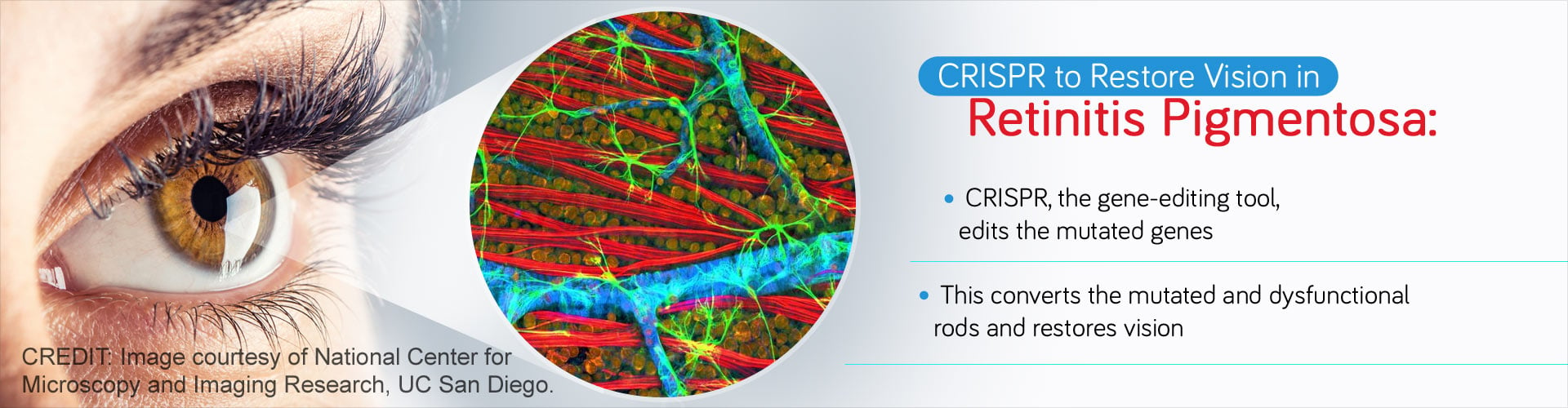
Advanced gene editing tool, CRISPR/Cas9, was used to reprogram photoreceptor mutated rods to functioning cones and restore vision in retinitis pigmentosa.
Highlights
- Retinitis pigmentosa causes genetic mutations in the retinal rod photoreceptor cells that leads to its dysfunction and degeneration over time, causing blindness eventually.
- The CRISPR/Cas9 gene-editing tool was used to deactivate a master switch gene called Nrl and a transcription factor called Nr2e3.
- Deactivating either Nrl or Nr2e3 helped to reprogram the mutated rod cells to functioning cone cells, which helped reverse degeneration and restore vision.
The mutations affect the photoreceptors in the eyes. Photoreceptors are specialized cells in the retina that sense and convert light images into electrical signals sent to the brain.
There are two types:
- rod cells for night vision and peripheral vision
- cone cells for central vision (visual acuity) and distinguishing colors
Using CRISPR/Cas9
RP is characterized by rod-specific genetic mutations that causes rod photoreceptor cells to dysfunction and degenerate over time. Over time, cone cells are also affected and they begin to fail and die.
The research team was led by senior author Kang Zhang, MD, PhD, chief of ophthalmic genetics, founding director of the Institute for Genomic Medicine and co-director of biomaterials and tissue engineering at the Institute of Engineering in Medicine, both at UC San Diego School of Medicine.
CRISPR, allows researchers to target specific stretches of genetic code and edit DNA at precise locations, modifying select gene functions.
"Cone cells are less vulnerable to the genetic mutations that cause RP," said Zhang. "Our strategy was to use gene therapy to make the underlying mutations irrelevant, resulting in the preservation of tissue and vision."
This approach was tested in two different mouse models of RP. This resulted in an abundance of reprogrammed cone cells and preserved cellular architecture in the retinas. Electroretinography testing of rod and cone receptors in live mice show improved function.
"Human clinical trials could be planned soon after completion of preclinical study. There is no treatment for RP so the need is great and pressing. In addition, our approach of reprogramming mutation-sensitive cells to mutation-resistant cells may have broader application to other human diseases, including cancer." Zhang said.
The findings are published in the online issue of Cell Research.
Reference
- Kang Zhang et al. Gene and mutation independent therapy via CRISPR-Cas9 mediated cellular reprogramming in rod photoreceptors. Cell Research; (2017) doi: 10.1038/cr.2017.57
Source-Medindia