US based drug maker Wyeth suffered one more set back in recent months when a federal judge refused to block another firm from marketing a generic version of the Wyeth’s heartburn drug Protonix.
Generics have their supporters in the US too, it looks. For a federal judge in the state of New Jersey on Thursday refused to block the Israeli firm Teva Pharmaceutical Industries from marketing its generic version of Protonix, a popular heartburn drug.
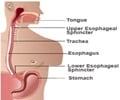
And mind you Protonix is manufactured by the US-based Wyeth Pharmacetuicals and its Protonix patent is not set to expire until July 2010. Still Teva sought to challenge its validity and, surprisingly, has won.Protonix, a so-called proton pump inhibitor that reduces stomach acid production, generated about $1.8 billion in revenue in 2006 and is Wyeth’s third-largest product. The drug, known generically as pantoprazole, is used to treat severe inflammation of the esophagus caused by gastric reflux (a burning sensation in the centre of the chest due to acidic stomach contents moving upwards into the esophagus).
Because the ruling by Judge Linares is under seal, Ken Cacciatore, an investment analyst for Cowen & Company, said it was not clear how strongly the judge had worded the ruling in Teva’s favor. “Teva would obviously be much more willing to launch if the ruling was predicated on a high likelihood of success on the merits,” Cacciatore said in a note to clients.
Wyeth, in a news release Thursday night, said that Judge Linares had emphasized that the findings were preliminary. The company said it believed that the Protonix patent was valid and that it would continue to fight the case.
Earlier in the summer the Wyeth had received two successive setbacks when the Food and Drug Administration said it needed more data before it could approve two of the company’s experimental drugs: Pristiq for menopausal symptoms and bifeprunox for schizophrenia.
Source-Medindia
SRM /J