Generic drug makers are racing to win FDA approval for selling their heartburn drugs on the market.

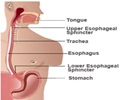
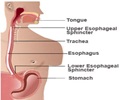
Esomeprazole, the chemical name of Nexium, essentially represents a proton pump inhibitor that cures acid reflux, formally known as Gastro-Esophageal Reflux Disease (GERD).
The drug is also known for reducing occurrence of potential gastric ulcers. The use of non-steroidal anti-inflammatory medications deals with various stomach infections and cancers caused by the bacterium Helicobacter pylori, and with other acid indigestion indications.
Gastro-Esophageal Reflux or acid reflux reflects an indication, where the contents moves back to the esophagus as there is acid indigestion by the stomach.
The disease leads to further complications including respiratory issues and esophageal inflammation.
The International Foundation for Functional Gastrointestinal Disorders (IFFGD) reports that about 5-7% of the worldwide population has some symptoms of GERD, with patients complaining of frequent heartburn.
Advertisement