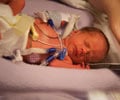
The German firm that made thalidomide has issued its first apology in 50 years to the thousands born disabled as a result of the drug's use, drawing stinging criticism from advocates

An estimated 10,000 children worldwide were born with defects -- including missing limbs -- after their mothers took thalidomide, which was sold in nearly 50 countries before being pulled from the market in 1961.
In a translated copy of the German text published on Grunenthal's website, Stock said he wanted to express his company's "sincere regrets" and "deep sympathy" to all those affected, "their mothers and their families",
"We ask that you regard our long silence as a sign of the shock that your fate has caused us," he said in the speech.
"We also apologize for the fact that we have not found the way to you from person to person for almost 50 years. Instead, we have been silent and we are very sorry for that."
Stock delivered the speech at the inauguration of a special memorial to thalidomide victims in Stolberg, western Germany, where the company is based.
Advertisement
The apology was rejected as insufficient by the charity Thalidomide Agency UK, which represents people affected by the drug in Britain.
Astbury, who was born in Chester in 1959 without arms or legs after his mother took the drug, said: "If they are serious about admitting they are at fault and regret what happened they need to start helping those of us who were affected financially."
Stock said his firm was taking steps to help the victims of the drug's use.
"We have learned how important it is that we engage in an open dialogue with those affected and to talk and to listen to them," he said.
Lawyers for Australian survivors of the drug Saturday described the belated apology from the firm which developed and marketed the drug as "pathetic" and "insulting".
"The apology issued by Grunenthal to thalidomide survivors is pathetic: it is too little, too late and riddled with further deceit," lawyers for Australian survivor Lynette Rowe said in a statement.
"To suggest that its long silence before today ought to be put down to 'silent shock' on its part is insulting nonsense.
"For 50 years Grunenthal has been engaged in a calculated corporate strategy to avoid the moral, legal and financial consequences of its reckless and negligent actions of the 1950s and 1960s."
Rowe's lawyers, from Gordon Legal and Slater & Gordon, said it would have been better for Grunenthal to release its private records to the world, including those it recently handed over in a class action led by the Australian woman.
Court papers used in the case brought by Rowe, who was born without arms or legs after her mother took thalidomide, allege that the German makers of the drug were warned of birth defects some two years before it was withdrawn.
Rowe's lawyers said Saturday the case against Grunenthal, which has denied wrongdoing and vowed to "fully defend" any legal action, was on hold after she reached a multi-million dollar settlement with another firm that had distributed the drug.
But they said lawsuits recently commenced in Australia would be followed up in many other countries.
A Japanese supporting group for Thalidomide victims also said an apology was not good enough.
"An apology is a matter of course," said Tsugumichi Sato, director-general of "Sakigake" Thalidomide Welfare Centre, in Japan, one of the major countries victimised of the drug disaster after Germany and Britain.
"The number of victims would have been smaller if the company had stopped its sales earlier," Sato said. "We are closely watching what responsibility the company will take on top of apologies."
Source-AFP