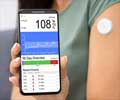
The device can be used for close monitoring of blood glucose levels in critically ill patients every 15 seconds through an automated calibration module.
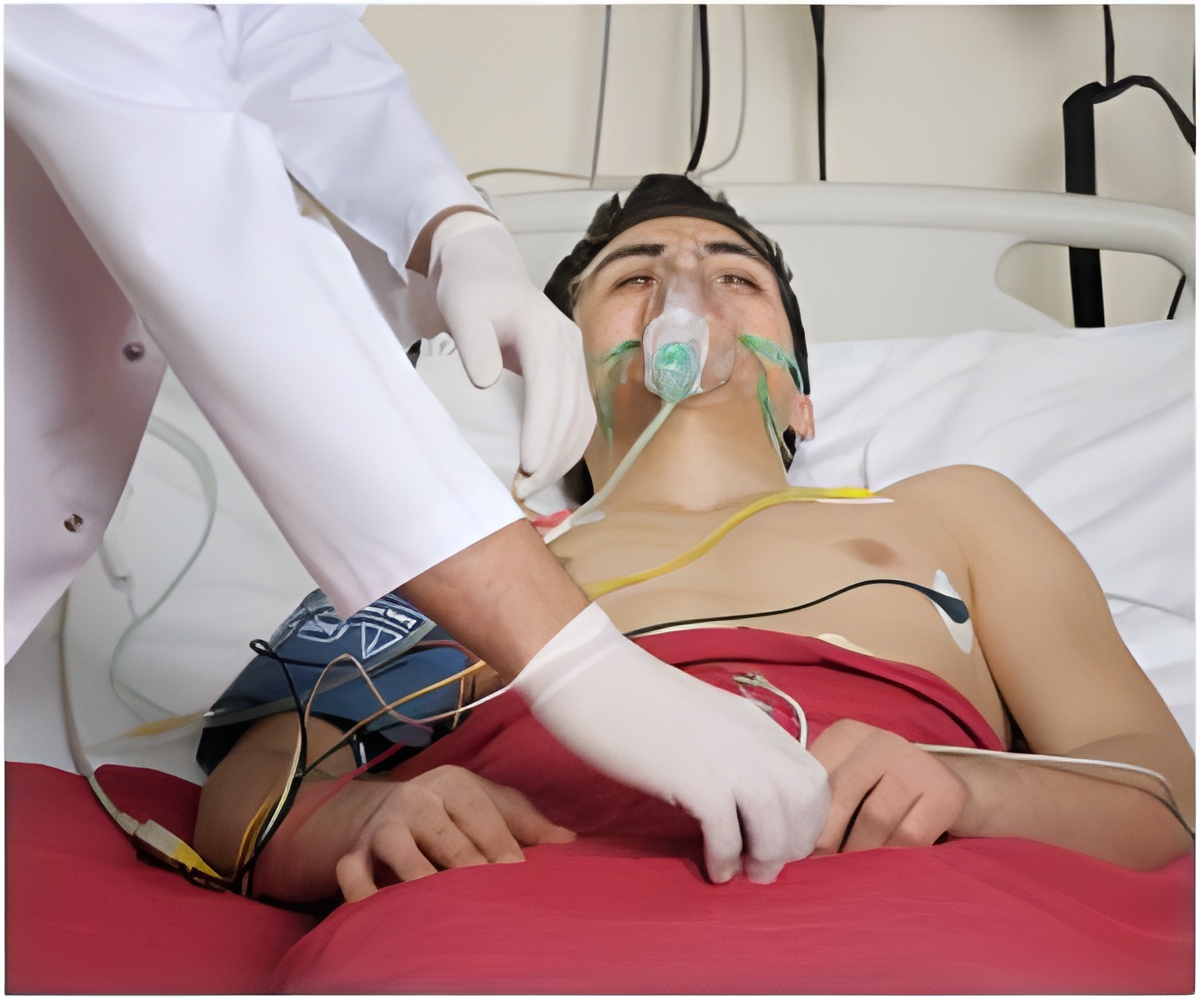
Before the system is used, a calibration module is used to prepare the sensor. It’s sterile and comes pre-connected to the fully automated sensor.
Once ready, the catheter and sensor is inserted into the patient, the system is activated and the sensor begins taking readings every fifteen seconds, displaying them on the screen.
The company is now working on finalizing a clinical trial that will be used to demonstrate the system’s safety and efficacy to the FDA in order to seek approval in the US.
Source-Medindia