Both microbial and host inflammatory factors modulate sulfomucin production in a human cell line, LS174T, that models intestinal goblet cells, a team of scientists has demonstrated
The research team at the University of Illinois compared the effects of bacterial flagellin to the mucogenic cytokine IL-13, and the proinflammatory cytokine TNF on expression of genes encoding the Golgi sulfotransferases involved in addition of sulfate groups to mucin precursors and sulfomucin production. They observed high induction of carbohydrate (N-acetyl-glucosamine 6-O) sulfotransferase 5 (CHST5) as early as 12 h after treatment with IL-13. Flagellin, IL-13 and TNF all, on the other hand, induced expression of the other sulfotransferase galactose-3-O-sulfotransferase 2 (GAL3ST2). The observed induction of sulfotransferases was consistent with increased sulfomucin production by LS174T cells in response to IL-13 and flagellin, indicating that sulfotransferases and sulfomucin synthesis can be differentially modulated by particular inflammatory signals.
Galactose-3-O-sulfotransferase 2 is thought to be the sulfotransferase responsible for sulfate addition to the C-3 position of galactose in human colonic mucins. On the other hand, CHST5 is the most likely candidate for sulfation of the C-6 position of N-acetylglucosamine. GAL3ST2 expression was enhanced in LS174T cells following treatment with flagellin, IL-13 and TNF, indicating that increased mucin sulfation at the C-3 position of galactose might be a common response to inflammatory stimuli.
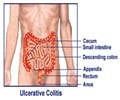
Although cautious to point out that the present study derives from a well-differentiated, but transformed goblet cell line, Professor Gaskins said that "the data implicate biosynthesis of sulfomucins as a potential therapeutic target for the restoration of barrier function in chronic intestinal inflammatory disorders. Recent studies provide strong evidence that both ulcerative colitis (UC) and Crohn's disease (CD), the two primary types of IBD, result from multifactorial interactions among genetic, environmental and immunological factors that lead to a dysregulation of the innate immune response to the intestinal microbiota in genetically predisposed individuals."
The results with TNF are of particular interest because of its association with IBD and the alterations in mucin sulfation seen with active IBD. As IBD is characterized by dysregulated immune responsiveness, it may be that individuals with IBD fail to respond appropriately to TNF and, hence, fail to increase the production of sulfomucins. However, much additional work will be needed to better understand the mechanisms by which flagellin, IL-13, and TNF enhance expression of specific sulfotransferases and sulfomucin production.
Dr. Steve Goodman, Editor-in-Chief of Experimental Biology and Medicine said "Crohn's disease and ulcerative colitis (UC), the two primary types of inflammatory bowel disease (IBD), afflict 0.1-0.5% of individuals in Western countries with approximately 1 million Americans suffering from the disease at a cost of over $2 billion. This impressive study by Gaskins and colleagues identifies sulfomucins as potential targets for future therapies for chronic intestinal inflammatory disorders".
Advertisement