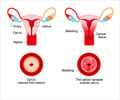
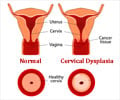
The Indian government has ordered that the cervical cancer vaccine trials in Gujarat and Andhra Pradesh must be stopped after the death of six tribal girls
The Indian government has ordered that the cervical cancer vaccine trials in Gujarat and Andhra Pradesh must be stopped after the death of six tribal girls who were administered the vaccine. The government said that permission was indeed given for the vaccine trials, but that the unfortunate incident called for further investigation.
S.Gandhiselvan, minister of state for health and family welfare, said in a statement to the Lok Sabha that two imported brands of the Human Papilloma Virus (HPV) vaccines, namely Gardasil and Cervarix, were allowed to undergo clinical trial (Phase III) in India."To assess the need of health services and the preparedness for introducing the Human Papilloma Virus (HPV) vaccines in the future, PATH, an international NGO, was given permission for a post licensure observational study of HPV vaccination in Khammam district (Andhra Pradresh) and Vadodara district (Gujarat). The study by PATH was recommended by the Ethical Committee and Advisory Groups at the state and central level," he revealed.
Four of the six deaths occurred in Andhra, while the other two occurred in Gujarat. Although the cause of deaths was determined as viral fever, drowning, suicide, severe anaemia with malaria and suspected snake bite, the government has decided to stop vaccine trials.
Source-Medindia
RAS