Evidence on whether or not there is any significant difference between the two current surgical approaches to treat patients with severe ischemic mitral regurgitation was reported by researchers.
"Ischemic mitral regurgitation is an important public health problem affecting as many as two million Americans. Our study is the first definitive clinical trial to address the relative merits of mitral valve repair versus mitral valve replacement surgery in patients suffering from severe ischemic mitral regurgitation," says Annetine C. Gelijns, PhD, Professor and Chair of the Department of Health Evidence and Policy at Icahn School of Medicine at Mount Sinai, who is the corresponding author for the NEJM study and principal investigator for the Data and Clinical Coordinating Center based at Mount Sinai for the NIH-sponsored Cardiothoracic Surgical Trials Network (CTSN) which conducted the clinical trial study.
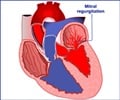
Ischemic mitral regurgitation occurs when blood backflows into the left atrium from the left ventricle of the heart due to improper closure of the mitral valve. The condition often develops as a complication from a heart attack and subsequent enlargement of the left ventricle, the heart's main pumping chamber. If not surgically corrected, the condition has been reported to potentially have a one-year mortality risk as high as 40 percent.
Currently, practice guidelines recommend valve repair or replacement for severe ischemic mitral regurgitation. However, lack of evidence has led to uncertainty and variation in surgical practice, although surgeons' use of repair has increased over time. Previous research has shown mitral valve repair has lower operative mortality and complications, while mitral valve replacement may provide better long-term correction of mitral regurgitation.
"These results may impact the surgical care of patients experiencing severe ischemic mitral regurgitation and offer the strongest evidence to date to inform future surgical practice guidelines," says Michael K. Parides, PhD, Professor of Biostatistics in the Department of Health Evidence and Policy at Icahn School of Medicine at Mount Sinai, the principal statistician for the CTSN.
This multicenter, randomized clinical trial included 22 clinical centers in North America randomizing 251 patients. It evaluated the safety and effectiveness of mitral valve repair and mitral valve replacement in patients with severe ischemic mitral regurgitation. Comparative effectiveness was based on the degree of left ventricular remodeling, mortality, adverse events, recurrent mitral regurgitation, hospitalizations, and quality of life one-year after surgery.
Advertisement
The CTSN has eight core clinical centers in the U.S. and Canada. The CTSN Data and Clinical Coordinating Center, based at Icahn School of Medicine at Mount Sinai, directs each of its clinical trial's design, methodologies, ethical research concerns, study data, analysis, and reporting.
Advertisement