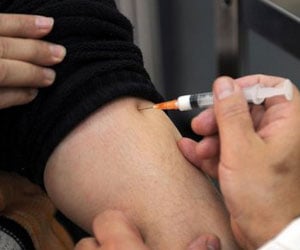
The efficacy of 2009 H1N1 vaccine in kids aged of 10 to 17 is promising, according to initial results of preliminary trials.
The efficacy of 2009 H1N1 vaccine in kids aged of 10 to 17 is promising, according to initial results of preliminary trials.
The researchers found that a single 15-microgram dose of a non-adjuvanted 2009 H1N1 influenza vaccine - the same dose that is in the seasonal flu vaccine - generates an immune response that is expected to be protective against 2009 H1N1 influenza virus in the majority kids within 8 to 10 days after vaccination."This is very encouraging news," said NIAID Director Dr Anthony S. Fauci.
"As we had hoped, responses to the 2009 H1N1 influenza vaccine are very similar to what we see with routinely used seasonal influenza vaccines made in the same way.
"It seems likely that the H1N1 flu vaccine will require just one 15-microgram dose for children 10 to 17 years of age. The 2009 H1N1 influenza virus is causing widespread infections among children, so these are welcome results," he added.
The ongoing trial, sponsored by National Institute of Allergy and Infectious Diseases, is assessing the safety and immune responses to one and two doses of either 15 micrograms or 30 micrograms of vaccine.
The study showed that immune responses were strongest among the oldest children, those 10 to 17 years old after first vaccination.
Advertisement
In the youngest group, 20 children between 6 months to 35 months old, a single 15-microgram dose of vaccine produced a strong immune response in 25 percent of recipients.
Advertisement
The vaccine being tested in this trial is manufactured by Sanofi Pasteur in Swiftwater, Pa., in the same manner as its licensed seasonal vaccine.
Like inactivated seasonal influenza vaccines, the vaccine contains a purified part of a killed virus and cannot cause flu.
Source-ANI
RAS