A type of antidiabetic drug called dipeptidyl peptidase-4 inhibitors are associated with the incidence of inflammatory bowel disease in patients with type 2 diabetes. Physicians should be made aware of this possible association.
- Certain diabetes drugs like dipeptidyl peptidase-4 inhibitors may increase the risk of inflammatory bowel disease
- Dipeptidyl peptidase-4 inhibitors are a new kind of diabetes drug prescribed to patients who do not respond to other diabetes drugs
- The findings need to be replicated, but physicians should be made aware of this possible association.
Dipeptidyl Peptidase-4 (DPP-4) Inhibitors
DPP-4 inhibitors are a new type of diabetes drug prescribed to lower high blood sugar levels in patients with type 2 diabetes who do not respond well to other antidiabetic drugs. These drugs block the DPP-4 enzyme that is involved in the body’s inflammatory response and regulate gut hormones. In patients with inflammatory bowel disease, low serum levels of the DPP-4 enzyme is linked to increased disease activity, according to recent studies. But no study has investigated the impact of inhibiting the enzyme on the occurrence of the disease.The research team analyzed data from the UK’s Clinical Practice Research Database involving 141,170 patients aged 18 years and above. The patients were on antidiabetic drugs between 2007 and 2016. The study excluded patients treated with insulin and those with a history of inflammatory bowel disease or similar digestive disorders. Patients’ age, body mass index (BMI), smoking status, alcohol-related disorders and complication of diabetes were taken into account. The study participants were monitored for an average of three and a half years. The research team found that during the monitoring period, 208 new cases of inflammatory bowel disease were recorded with an incidence rate of 37.7 per 100,000 person years.
The use of DPP-4 inhibitors was associated with an increased risk of inflammatory bowel disease by 75% (53.4 cases per 100,000 person years) compared with the use of other antidiabetic drugs (34.5 cases per 100,000 person years).
The association between DPP-4 inhibitor use and inflammatory bowel disease increased with longer durations of DPP-4, reaching a peak after three to four years and decreasing after more than four years of use.
The research team said that this is an observational study and no firm conclusions can be drawn on the cause and effect. The limitation of the study is the possible misclassification of drugs or cases. The other limitation is after adjusting for several risk factors; the research team could not rule out the possibility that other unmeasured factors may have influenced the results. Nevertheless, this is the first population-based study to analyze the use of DPP-4 inhibitors association with an increased risk of inflammatory bowel disease.
The research team concluded that “although our findings need to be replicated, physicians should be aware of this possible association and perhaps refrain from prescribing DPP-4 inhibitors for people at high risk for inflammatory bowel disease, such as those with a family history of the disease or with known autoimmune conditions.”
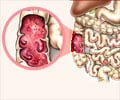
What is Inflammatory Bowel Disease?
Inflammatory bowel disease is a term for Crohn's disease and ulcerative colitis that is characterized by chronic inflammation of the gastrointestinal tract. Inflammatory bowel disease causes abdominal discomfort and bloating.Reference:
- Azoulay L, Bitton A, Renoux C, Yun Yu O H, Yin H, Douros A, Abrahami D. “Dipeptidyl peptidase-4 inhibitors and incidence of inflammatory bowel disease among patients with type 2 diabetes: population based cohort study”, The BMJ (2018). doi: (https://doi.org/10.1136/bmj.k872)
Source-Medindia