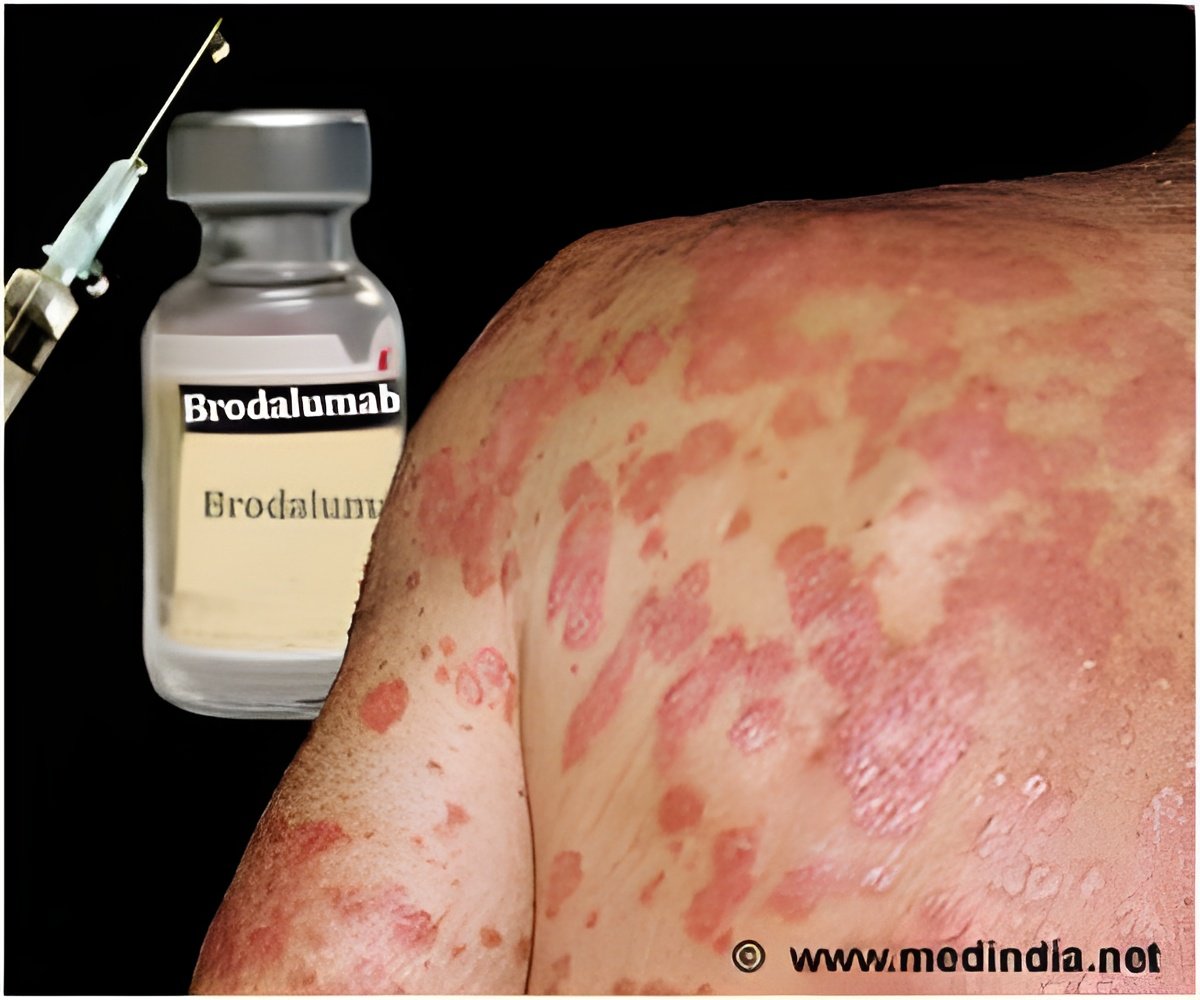
A new drug, brodalumab appears to be effective in moderate-to-severe psoriasis.
The interleukin-17 group consists of 6 cytokines from A to F. These act on 5 receptors, interleukins 17RA to 17RE, to bring about their effect.
Brodalumab is a monoclonal antibody that inhibits the interleukin-17 pathway. It blocks the action of 17A, 17F, 17A/F heterodimer, and 17E. A recently published study assessed the short-term efficacy and safety of brodalumab in patients with moderate-to-severe plaque psoriasis.
The study was an international study in which patients received either placebo or brodalumab at a dose of 70 mg, 140 mg, or 210 mg, subcutaneously on day 1 and at weeks 1, 2, 4, 6, 8, and 10, or at a dose of 280 mg subcutaneously on day 1 and at weeks 4 and 8.
A total of 188 patients completed the study. Patients taking brodalumab showed significant improvement in psoriasis as compared to those who did not receive the medication. The improvement was observed as early as 2 weeks after starting treatment. The improvement in psoriasis was significantly better in patients taking 140 mg, 210mg and 280 mg brodalumab as compared to 70 mg.
The quality of life was less affected in people taking brodalumab as compared to those not taking the drug. The physical component of well being was increased significantly in the 140 mg group whereas the mental component was significantly better in the 140mg and 210 mg groups compared to those not taking the medication. Improvements were also observed in biopsy specimens from patients undergoing treatment with brodalumab.
Common adverse effects observed in the study included nose and throat inflammation, upper respiratory tract infection, joint aches, and redness at the injection site. Serious adverse effects included renal colic in 1 patient, an ectopic pregnancy (pregnancy outside the uterus) in 1 case and asymptomatic neutropenia (decrease in white blood cell count) in 2 cases.
Reference:
1. Brodalumab, an Anti–Interleukin-17–Receptor Antibody for Psoriasis; Kim Papp et al; N Engl J Med 2012; 366:1181-1189March 29, 2012
Source-Medindia