Novel regimen combining chemotherapy and targeted molecular therapy vastly improves response and patient outcome in younger chronic lymphocytic leukaemia patients
Highlights:
- Patients less than 65 years diagnosed with chronic lymphocytic leukaemia (CLL) showed significantly better response when treated with a combination of chemotherapy and targeted molecular therapy
- Average age of diagnosis in CLL is over 70 years; however elderly patients do not tolerate chemotherapy very well
Novel Ibrutinib–FCR (iFCR) Treatment Regimen For Younger CLL Patients
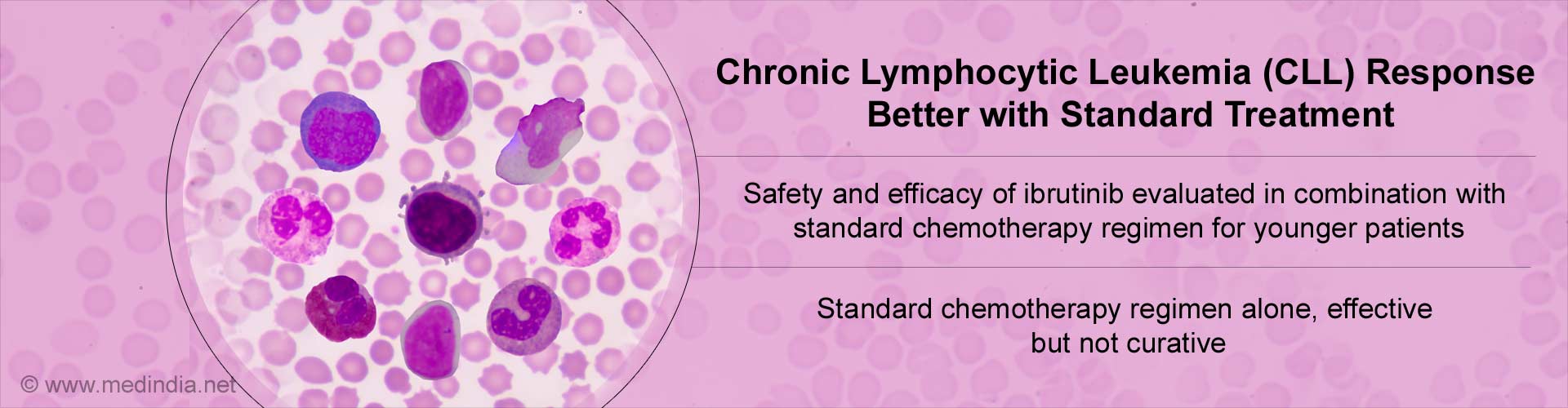
- Chronic lymphocytic leukaemia (CLL) is a blood disorder in the elderly with average age at diagnosis being about 72 years.
- Since older patients do not tolerate chemotherapy well due to toxicities and adverse effects, the combination of Fludarabine, cyclophosphamide, and rituximab (FCR) is the standard chemoimmunotherapy regimen used for younger patients with CLL.
- Although this treatment is very effective in controlling the disease, it does not cure CLL.
- In the current study, the research team combined ibrutinib a newer agent, with the standard FCR chemotherapy regimen for CLL to determine whether this combination (iFCR) is safe and more effective for younger patients with CLL.
Methods Of The Study
- The study included only physically fit patients 65 years or younger and who had not received any prior treatment, for this trial.
- Participants of the trial initially got approximately six months of a combination of FCR (fludarabine, cyclophosphamide, and rituximab) chemotherapy along with the targeted oral agent ibrutinib.
- This regimen was then followed by ongoing maintenance with ibrutinib.
How Superior Is The Novel Treatment Regimen - Findings Of The Study
- All of the 35 patients who received this "iFCR" combination responded to this treatment
- Following the end of the initial part of the treatment with iFCR, 37 percent obtained complete response with no evidence of minimal residual disease (MRD) in the bone marrow, compared to the 20 percent response with FCR alone
- Following initial treatment, during the maintenance period, the proportion of MRD-negative complete response increased to 57%, with 83% showing nil residual disease in the bone marrow
- Patients given iFCR treatment showed relatively lower rates of infection and blood toxicity (although this could also be due to mandatory prophylaxis with antimicrobials and growth factor support given to these patients)
- However, in this trial even among patients with the high-risk unmutated IGHV gene, about 71 percent showed no evidence of residual disease in the bone marrow.
Thus the findings of the study suggest that iFCR combination regimen achieves better and more lasting response in CLL patients compared to FCR alone.
"Previous studies have demonstrated that when we use ibrutinib alone, it is very effective at controlling the disease but it doesn’t eradicate it," Davids said. "A goal in our field now is to try to combine targeted agents such as ibrutinib with other drugs."
Novel Agent Ibrutinib – An Overview
Ibrutinib is a BTK (Bruton Tyrosine Kinase) inhibitor, and blocks this protein which is necessary for CLL cells to survive. It was approved by the Food & Drug Administration (FDA) for the treatment of newly diagnosed CLL in 2016. It is administered orally.It is used to treat chronic lymphocytic leukemia, Waldenstrom’s macroglobulinemia, and as a second-line treatment for marginal zone lymphoma, mantle cell lymphoma, and chronic graft vs host disease. Common side effects of Ibrutinib include the following
- Decreased platelets causing easy bruising and bleeding
- Nausea, vomiting and diarrhea
- Decreased neutrophil count
- Decreased hemoglobin
- Tiredness
- Musculoskeletal pain
- Swelling
- Upper respiratory tract infection
Future Plans
- To enlarge upon the current study, it is planned to recruit 50 patients more supported by the Blood Cancer Research Partnership, a research consortium codirected by Davids with started with funds given from the Leukemia and Lymphoma Society.
- In that study, the participants will stop getting ibrutinib maintenance after two years if they are MRD negative in the bone marrow.
References:
- A Phase II Study of Ibrutinib Plus FCR in Previously Untreated, Younger Patients With Chronic Lymphocytic Leukemia (iFCR) - (https://clinicaltrials.gov/ct2/show/NCT02251548)
- About Ibrutinib - (http://chemocare.com/chemotherapy/drug-info/ibrutinib.aspx)