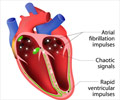
The direct oral anticoagulants edoxaban, apixaban, rivaroxaban and dabigatran have been recently approved for the treatment of non-valvular atrial fibrillation
- The new direct oral anticoagulants (DOACs) have been approved for the treatment of non-valvular atrial fibrillation
- DOACs do not require regular monitoring with INR unlike warfarin
- DOACs dose should be reduced in kidney dysfunction
Warfarin has been the only oral anticoagulant available for a very long time.
- It requires frequent monitoring of its effect through the measurement of the International Normalized Ratio (INR)
- It interacts with other medications
- It can cause fetal malformation when used in pregnancy
The DOACs can be used in those patients in whom the INR cannot be maintained within the desired range. They do not require monitoring of INR and can be administered as a fixed dose. However, kidney function should be regularly monitored in patients taking these medications. It is also important to note that specific antagonists to these drugs are not yet available. Prothrombin complex concentrate has sometimes been used to reverse the anticoagulation caused by the medications. Idarucizumab has recently been approved for the treatment of serious bleeding following the use of dabigatran.
A summary of the important information derived from the article published in the US Pharmacist by Hoie EB and colleagues is presented below:
Edoxaban
- Edoxaban is available as a tablet to be taken in a dosage of 60 mg per day for a patient with non-valvular atrial fibrillation
- Since most of it is excreted from the body through the urine, the dosage has to be reduced in those with reduced kidney function. It should not be used by patients with severe kidney disease and mild-to-moderate liver disease. It belongs to pregnancy Category C and should not be used during breastfeeding
- The use of drugs like rifampin, other anticoagulants and antiplatelet drugs which could alter the effect of edoxaban should be avoided in a patient taking edoxaban
- As expected, side effects are bleeding and anemia
Apixaban
- Apixaban is used in a dosage of 5 mg twice a day. The dosage should be reduced in individuals with severe kidney disease, over 80 years of age, and in patients with body weight of less than 60 kg
- It can be used during pregnancy (Pregnancy category B), but can increase the risk of bleeding. It should not be used by nursing mothers or those with severe liver disease
- It should not be used with drugs that may alter its metabolism or its blood levels
Rivaroxaban
- Rivaroxaban is used in a dose of 20 mg a day with the evening meal. Dosage should be reduced in patients with kidney disease, and should be avoided in patient with severe kidney disease.
- It belongs to Pregnancy Category C and should not be used in nursing mothers
- It should not be used in patients with moderate-to-severe liver disease
- It should not be used with drugs that may alter its metabolism or its blood levels
Dabigatran
- Dabigatran is used in a dose of 150 mg twice daily, which should be reduced in patients with kidney disease
- It belongs to Pregnancy Category C and should not be used by nursing women
- It commonly causes gastritis-like adverse events
- Hoie EB, O’Brien KK, Neighbors K, Castillo SL, Begley KJ. Direct Oral Anticoagulants for the Prevention of Stroke in Nonvalvular Atrial Fibrillation. US Pharm. (2017);42(2):32-35.
Source-Medindia