- The study compared prostate cancer mortality rate between subjects invited for a single invitation screening study for Prostate-specific antigen (PSA) and those with no invitation for PSA testing
- The CAP clinical trial data indicated that prostate cancer mortality at a median follow-up of 15 years was lesser in groups with single invitation as compared to those with no invitation, but the benefit was small
- The absolute reduction in deaths due to prostate cancer in screened patients was small
Prostate-Specific Antigen Screening and 15-Year Prostate Cancer Mortality: A Secondary Analysis of the CAP Randomized Clinical Trial
Go to source). In a recent study, Martin et al conducted a secondary analysis clinical study to evaluate the effect of a single invitation for Prostate-specific antigen (PSA) screening on prostate cancer-specific mortality at a median 10-year follow-up, as compared to a study with no invitation for PSA screening.
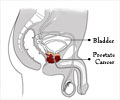
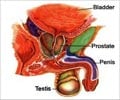
Prostate cancer ranks second as the most commonly occurring cancer and the fifth most important cause of cancer death among men worldwide. #Prostate #Cancer #medindia’
Prostate Cancer Trial: Study Design and Subjects
The study was a secondary analysis of the Cluster Randomized Trial of PSA Testing for Prostate Cancer (CAP). The primary aim of this study was to assess whether a single PSA screening invitation could reduce prostate cancer-specific mortality compared to no invitation.This clinical study was conducted on men aged between 50 to 69 years, from primary care centers in England and Wales. These men were randomly assigned to either receive a single invitation for PSA screening or standard care without screening.
The study meticulously followed participants for a median of 15 years, evaluating outcomes such as prostate cancer mortality, all-cause mortality, and cancer stage and grade at diagnosis.
The enrollment of men was done between January 8, 2002, and January 20, 2009; the follow-up was completed on March 31, 2021.
Study on PSA Screening of Prostate Cancer: Results and Implications
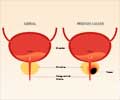
The study included 415,357 men, of which 195,912 men belonged to the single invitation PSA Screening group, and 219,445 men to the control group, without PSA screening. At the median follow-up period of 15 years, 1,199 men (0.69%) in the single invitation group and 1,451 men in the control group (0.78%) died of prostate cancer.Additionally, the screening intervention led to increased detection of low-grade and localized prostate cancers but did not significantly impact intermediate or high-grade tumors.
With prostate cancer ranking as the second most commonly diagnosed cancer and the fifth leading cause of cancer-related deaths among men globally, understanding the role of PSA screening in mitigating its impact is paramount. This report serves as a vital contribution to the ongoing discourse surrounding prostate cancer management, offering valuable insights into the effectiveness of PSA screening as a tool for reducing mortality rates and ultimately improving outcomes for men affected by this disease.
Reference:
- Prostate-Specific Antigen Screening and 15-Year Prostate Cancer Mortality: A Secondary Analysis of the CAP Randomized Clinical Trial - (https://pubmed.ncbi.nlm.nih.gov/38581198/)
Source-Medindia


![Prostate Specific Antigen [PSA] & Prostate Cancer Diagnosis Prostate Specific Antigen [PSA] & Prostate Cancer Diagnosis](https://images.medindia.net/patientinfo/120_100/prostate-specific-antigen.jpg)