HPV vaccine – A safe and effective treatment in prevention of cervical cancer.
Like for all other licensed medicines, the safety of these vaccines is being monitored by agency's Pharmacovigilance Risk Assessment Committee (PRAC). The present review will delve into the available data with focus on rare reports on two conditions: CRPS (complex regional pain syndrome), a chronic pain condition affecting the limbs and POTS (postural orthostatic tachycardia syndrome), a condition where heart rate increases abnormally following sitting or standing up, thereby causing symptoms like dizziness, fainting, headache, chest pain and weakness.
The above conditions have been previously reported in young women who have received an HPV vaccine. The report was then considered by the PRAC during routine safety monitoring. But, no causal relationship was established between the conditions and vaccines taken. These conditions can however also occur in non-vaccinated individuals too and it is therefore considered imperative for further review to assess if number of cases reported with HPV vaccine is greater than expected.
The review of HPV vaccines has been commenced by the European Commission at the request of Denmark, under Article 20 of Regulation (EC) No 726/2004. The review would be carried out by PRAC (the Committee responsible for the evaluation of safety issues for human medicines), who will be making a set of recommendations. In its review, PRAC will be considering the latest scientific knowledge, including any research that would help to elucidate the frequency of CRPS and POTS after vaccination or identify causal link if any. Based on this review, the committee would decide to recommend any changes to product information so as to better inform patients and healthcare professionals. However, under ongoing review there is no change in recommendations in use of the vaccines.
The recommendations made by PRAC will then be forwarded to the Committee for Medicinal Products for Human Use (CHMP), which is responsible for questions related to medicines for human use. The CHMP will then adopt a final opinion. The concluding stage of this review process would be decision adoption by the European Commission, which would be applicable to all European member states.
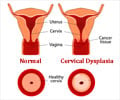
What are Human Papillomaviruses?
Human papillomavirus (HPVs) constitute a group of more than 200 related viruses. HPV of more than 40 different types can be easily transferred by direct sexual contact from skin and mucous membranes of people who are infected to the skin and mucous membranes of their spouse/partners. In addition, it can be spread by vaginal, anal and oral sex. Other HPV types responsible for non-genital warts are not sexually transmitted.About the Vaccines
How do HPV Vaccines Act?
HPV vaccines work by stimulating the antibody production. HPV vaccines are based on virus-like particles (VLPs) formed by HPV surface components. VLPs are not infectious as it lacks the viral DNA, but, they look like natural virus. Antibodies produced against VLPs act against the natural virus. VLPs are also immunogenic, as it induces a high level of antibody production, thus making vaccines highly effective.Safety & Efficacy of HPV Vaccines
As of now no serious side effects have been observed. Most common problems are brief soreness and local symptoms at the injection site. These vaccines are contraindicated in pregnancy as pertinent safety data is currently unavailable. Patients using Gardasil vaccine have reported a higher proportion of syncope (fainting) and venous thrombotic events (blood clots) as compared to other vaccines. It was also seen that the patients who developed blood clots had known risk factors for their development, such as the use of oral contraceptives. However, safety review of this particular vaccine in Denmark and Sweden did not identify an increased risk of blood clots. HPV vaccines are highly effective in infection prevention for the types of HPV it is being targeted.
References:
1. European Medicine Agency. Accessed July 24 2015. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2015/07/news_detail_002365.jsp&mid=WC0b01ac058004d5c1
2. Human Papillomavirus (HPV) Vaccines. National Cancer Institute. Accessed July 24 2015. Available from: http://www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-vaccine-fact-sheet
Source-Medindia