Tecentriq (Atezolizumab) from Roche shows significant potential as first-line therapy for the treatment of bladder cancer by improving survival.
- Bladder cancer is the ninth most common cancer in the world with limited new major treatment options in the past 30 years.
- FDA has approved Tecentriq (Atezolizumab), a monoclonal antibody from Roche, for the treatment of bladder cancer.
- Tecentriq (Atezolizumab) is found to shrink tumors and benefit the survival rate of the people with bladder cancer. It is expected to dominate the market for bladder cancer treatment.
Bladder Cancer and Tecentriq (Atezolizumab)
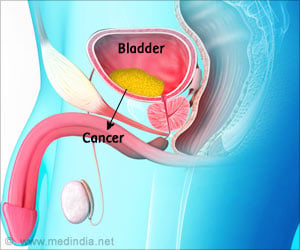
Bladder cancer refers to the growth of abnormal cancerous tissue or tumor in the lining of the urinary bladder. It is the 9th most common cancer in the world and has shown limited advancements in its treatment in the past few decades. It is responsible for approximately 145,000 deaths every year.Tecentriq (Atezolizumab) is a monoclonal antibody referred to as PD-1 (Programmed Death Ligand 1) inhibitor. It may bring about its action by allowing immune cells of the body to fight against the cancer. It has received FDA approval for locally advanced or metastatic urothelial carcinoma of the bladder that does not respond to or progresses despite treatment with platinum-based chemotherapy, the commonly used treatment for these cancers. It is given intravenously once every three weeks.
The drug has been studied in clinical trials in bladder cancer patients who have received platinum-based chemotherapy, or have not received any chemotherapy and has shown good response. It appears to shrink tumors and improve survival rate. Moreover, it can be used in patients who cannot receive platinum-based treatments like those with kidney failure.
Tecentriq, which is used as a second-line treatment for bladder cancer, has already started to expand its potential to become a first line therapy for bladder cancer.
Cai Xuan, an analyst in GlobalData which is a leading provider for data and analysis, feels that the complete responses of Tecentriq in the treatment of bladder cancer are significant since the disease is found to be almost fatal.
Demerits of Tecentriq
Adverse effects like urinary tract infections, anemia and fatigue are common with the use of Tecentriq (atezolizumab) for the treatment of bladder cancer. Breathing difficulties, abdominal pain, decreased appetite, sepsis, lung infections were also seen in some patients. It can also cause hormonal problems, and liver and large intestinal inflammation.- Drug Trials Snapshots: Tecentriq
http://www.fda.gov/drugs/informationondrugs/ucm504112.htm