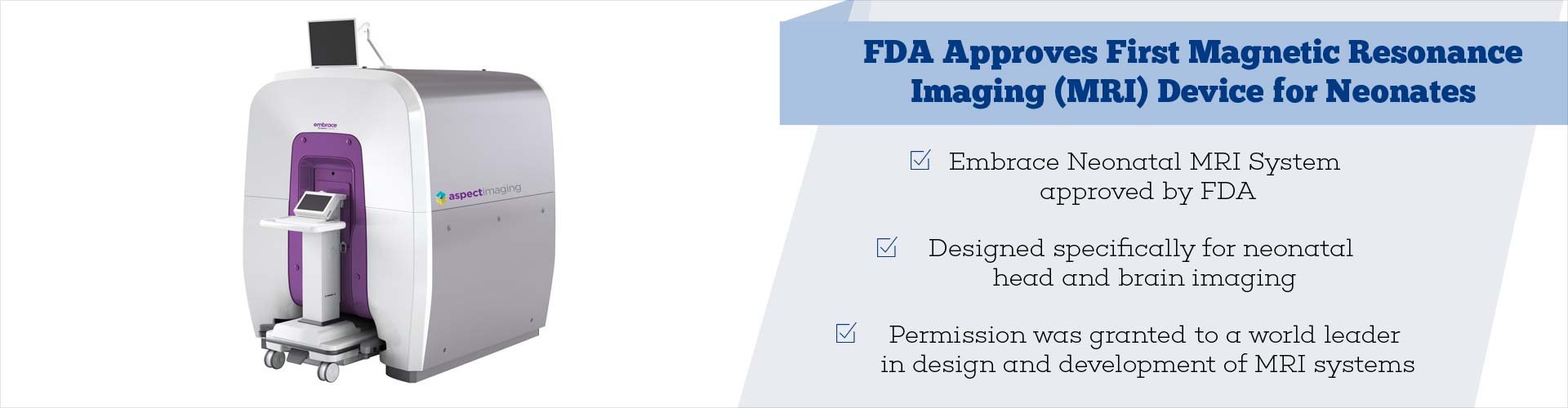
The Embrace Neonatal MRI System is the first magnetic resonance imaging device cleared by the FDA for use on neonates.
- The U.S. Food and Drug Administration cleared the first magnetic resonance imaging (MRI) device to be used on neonates
- Called the Embrace Neonatal MRI System, it is designed specifically for neonatal head and brain imaging
- This device can be placed inside the premises of neonatal intensive care units
An MRI procedure is a medical imaging procedure that is useful to diagnose as well as treat an array of conditions in the body. It uses strong magnetic fields, radio waves (radiofrequency energy) and a computer to produce detailed pictures of the internal structures of the body.
The radio waves manipulate the magnetic position of the atoms (mainly the protons in fat and water molecules in the body). The signals are picked up by a powerful antenna and sent to a computer. The computer performs millions of calculations, and produces clear, cross-sectional black and white images of the body from which three-dimensional (3-D) pictures are drawn of the scanned area. These pictures are then interpreted by a trained physician who diagnoses the condition.
Uses of an MRI
MRI devices can detect a variety of conditions including problems of the brain, spinal cord, chest, lungs, abdomen, pelvis, and limbs. An MRI of the brain in particular can be used to detect cysts, tumors, bleeding, swelling, developmental and structural abnormalities, infections, inflammatory conditions, or problems with the blood vessels. Sometimes it is used to determine the cause of symptoms like persistent headaches, dizziness, weakness, and blurry vision or seizures that may bog down the infant.
MRI is considered a fairly safe imaging technique with no evidence of serious harm to human tissue reported even when used on neonates. MRI devices are the only imaging procedures that provide clinicians and researchers objective, superior, in vivo information about brain anatomy, pathology and also functional and physiological characteristics in neonates.
Some of the general disadvantages of an MRI are the time required for preparation, complex scheduling, loud acoustic noise and high costs. But these can be overlooked because of the safety of an MRI and the fact that it can provide clear images of the organs that cannot be seen as well with an X-ray, CAT scan, or ultrasound.
A couple of drawbacks of using the MRI scanner seen particularly in neonates are the following. These can hopefully be eliminated in the Embrace Neonatal MRI System.
There should be no movement during an MRI as it can cause image artifact or distortion, prolong the examination and cause a misdiagnosis due to poor image quality. Since, it is not possible for infants to stay still, they are often sedated during MRI studies, which prolongs the time of imaging. Sedating agents could also pose a risk to the infant.
Also, up until now, neonates had to be transported to the rooms that housed the MRI scanners. This could expose them to infections. So having MRI scanners inside the premises of NICUs suitable is the need of the hour.
“Although we can use traditional MRI scanners to image neonates, taking babies outside of the neonatal intensive care unit to MRI suites presents great challenges,” said Vasum Peiris, M.D., M.P.H., chief medical officer for pediatrics and special populations at FDA’s Center for Devices and Radiological Health. “Having a system in the neonatal intensive care enables safer imaging for this vulnerable patient population.”
Embrace Neonatal MRI System
The Embrace Neonatal MRI System is designed specifically for imaging of the neonatal head.
Some of the features of the Embrace Neonatal MRI System are:
- It may be used on neonates who have a head circumference up to 38 centimeters and a weight of anywhere from 1 to 4.5 kilograms.
- It has a temperature-controlled incubator placed directly into the MRI system that helps in minimizing movement of the baby. If there is a need to access or remove the baby urgently during the imaging process, it can be done in less than 30 seconds.
- It can be placed directly inside a NICU environment since an MRI system is fully closed, and hence does not require a safety zone or a radiofrequency shielded room to protect other medical device implants in close proximity to the system.
- It cannot be used on patients who weigh more than 4.5 kilograms or who have a head circumference of more than 38 centimeters
- It cannot be used on infants with metallic or electronically active implants since it may heat up the tissue near the implant or cause the implant to malfunction.
The FDA granted clearance of Embrace Neonatal MRI System to Aspect Imaging Ltd, a world leader in design and development of MRI systems.
References:
- FDA clears first neonatal magnetic resonance imaging device - (https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm567840.htm)
- MRI fo the preterm infant - (http://www.pedrad.org/Portals/5/Events/2013/KlineFathPretermBrain-NEW.pdf)
- Fetal and Neonatal Brain Magnetic Rsonance Imaging - (http://www.bapm.org/publications/documents/guidelines/BAPM%20MRI%20standards%20for%20fetal%20neonatal%20brain%20imaging_FINAL%20SUBMISSION%20080216.pdf)
- Smyser CD, Kidokoro H, Inder TE. MRI of the brain at term equivalent age in extremely premature neonates – to scan or not to scan? Journal of paediatrics and child health. 2012;48(9):794-800. doi:10.1111/j.1440-1754.2012.02535.x.