FDA approves a drug for the first time for treating severe hypoglycemia. The drug, BaqsimiTM, can be administered nasally without an injection, which rapidly elevates blood sugar levels.
- FDA has approved a drug called Baqsimi™ for treating severe hypoglycemia
- It is the first drug for the treatment of severe hypoglycemia without the need for an injection
- It is administered nasally and its efficacy is as good as injectable glucagon, which is conventionally used for treating severe hypoglycemia
Read More..
What are the Dangers of Hypoglycemia?
Hypoglycemia is a condition that occurs primarily in diabetic patients that is characterized by a rapid fall in blood sugar levels below the normal range. Hypoglycemia is a medical emergency in patients with Type-1 or Type-2 diabetes. The hallmarks of hypoglycemia are altered mental and/or physical functioning, which requires immediate medical attention for recovery. In the absence of prompt treatment, it can lead to potentially life-threatening complications such as loss of consciousness, seizures, coma and even death.Presentation and Indications for the Use of Baqsimi™
Baqsimi™ is available as a compact, portable, ready-to-use pack that doesn’t require to be reconstituted. It has a single fixed dose of 3 mg powdered glucagon in the form of a nasal spray. The drug is absorbed directly through the nasal mucosa and therefore doesn’t need to be inhaled. Also, it doesn’t need to be refrigerated and can be kept at ambient temperatures (~30ºC). Baqsimi™is recommended in severe cases of hypoglycemia in diabetic adults and children aged 4 years and above.Mechanism of Action of Baqsimi™
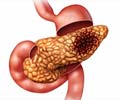
Baqsimi™works by stimulating the liver to release stored glucose into the bloodstream, which increases the blood sugar level. Its mechanism of action involves binding of glucagon with the glucagon receptors present in the liver. This stimulates gluconeogenesis, which is a metabolic process in which glucose is synthesized from non-carbohydrate precursors such as lactate, pyruvate and glucogenic amino acids. This newly synthesized glucose is rapidly released from the liver, thereby raising the blood sugar levels. This is essentially the exact opposite action of insulin, which lowers blood sugar levels.Advantages of Baqsimi™
Baqsimi™ has several advantages over injectable glucagon, which was approved by the USFDA way back in 1960. These advantages are well articulated by Dr. Janet Woodcock, MD, who is the Director of USFDA’s Center for Drug Evaluation and Research (CDER) at Silver Springs, Maryland, USA.“People who are living with diabetes are at risk of their blood sugar levels falling below the normal range. There are many products on the market for those who need insulin, but until now, people suffering from a severe hypoglycemic episode had to be treated with a glucagon injection that first had to be mixed in a several-step process,” said Woodcock. “This new way to administer glucagon may simplify the process, which can be critical during an episode, especially since the patient may have lost consciousness or maybe having a seizure. In those situations, we want the process to treat the suffering person to be as simple as possible.”
Clinical Trials of Baqsimi™
Clinical trials were carried out both in adults and children, which are briefly highlighted below:Adult Clinical Trials: Two clinical trials were carried out in adults, which included 83 and 70 adults, respectively. The safety and efficacy of Baqsimi™ nasal powder were evaluated head-to-head against injectable glucagon for treating insulin-induced hypoglycemia, using a single dose of each drug. Baqsimi™exhibited comparable efficacy to injectable glucagon in adults, with a 100 percent success rate in case of both drug formulations. The incidence of side-effects, which included headache, nausea, vomiting, and upper respiratory tract irritation, were similar in both cases.
Pediatric Clinical Trials: A clinical trial was carried out in children aged 4 years to <17 years. The clinical trial compared the safety and efficacy of Baqsimi™ and injectable glucagon. Both drugs exhibited a 100 percent success rate in elevating blood sugar levels, which was similar to that observed in adults. The incidence of side-effects was similar in both cases.
Contraindications for Baqsimi™
Baqsimi™ should not be taken in the following conditions:- Pheochromocytoma (rare adrenal gland tumor that secretes epinephrine and norepinephrine hormones)
- Insulinoma (insulin-secreting pancreatic tumor derived from β-cells)
- Hypersensitivity to glucagon
- Allergy to any excipients in Baqsimi™
- Prolonged fasting
- Chronic hypoglycemia
- Adrenal insufficiency
Side Effects of Baqsimi™
Some of the major side-effects include the following:- Headache
- Nausea
- Vomiting
- Upper respiratory tract irritation
- Nasal congestion / discomfort
- Coughing
- Sneezing
- Epistaxis (bleeding from the nose)
- Rhinorrhea (runny nose)
- Redness of the eyes
- Watery eyes
- Itchy eyes/nose
Conclusion
FDA approved drug Baqsimi™can help treat severe hypoglycemia without an injection. The drug is delivered nasally and results in rapid elevation of blood sugar levels.Reference:
- FDA approves first treatment for severe hypoglycemia that can be administered without an injection - (https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-severe-hypoglycemia-can-be-administered-without-injection)
Source-Medindia