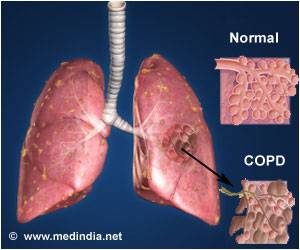
Formoterol is found to be effective and safe in the treatment of COPD.
The effects of formoterol and placebo in COPD patients were compared in a study recently published in BMC Pulmonary Medicine. The aim of the study was to establish the usefulness of the long-acting drug in COPD as well as to test its safety in COPD patients. Some prior studies had suggested that long-acting bronchodilators could increase the risk of death in these patients.
The study was carried out on a total of 613 patients of European and Japanese origin. Some patients received 4.5µg of formoterol twice daily, others received a doubled dose of 9µg of formoterol twice daily, and still others received a placebo (an inert substance for comparison purpose). The patients were administered the drug for a total duration of 12 weeks. Lung function was measured using a spirometer. Patients who needed additional medication could take the short-acting drug salbutamol whenever required. They could also continue to take short-acting anticholinergics as earlier.
At the end of the study period, it was found that the measured lung function (which was FEV1 60-min post-dose in the study) improved significantly in both the formoterol groups as compared to placebo. There was no significant difference between the two formoterol groups.
The improvement in clinically-relevant quality of life, as measured by a questionnaire, was significantly higher with formoterol 9µg as compared to placebo. However, there was no clinically-significant improvement in quality of life with 4.5µg formoterol as compared to placebo. Both groups on formoterol required less additional salbutamol treatment as compared to placebo; the group on the higher dose also required less salbutamol rescue as compared to the lower dose.
Most side effects in patients with formoterol were similar in number as compared to placebo. Side effects included nasopharyngitis (inflammation of the nose and throat), COPD exacerbation, and bronchitis complication. Two deaths occurred in the formoterol groups, but they were unrelated to the treatment.
Reference:
Source-Medindia