In a breakthrough discovery, scientists have found that by altering the faulty gene that causes Cystic Fibrosis (CF), the disorder can be treated.
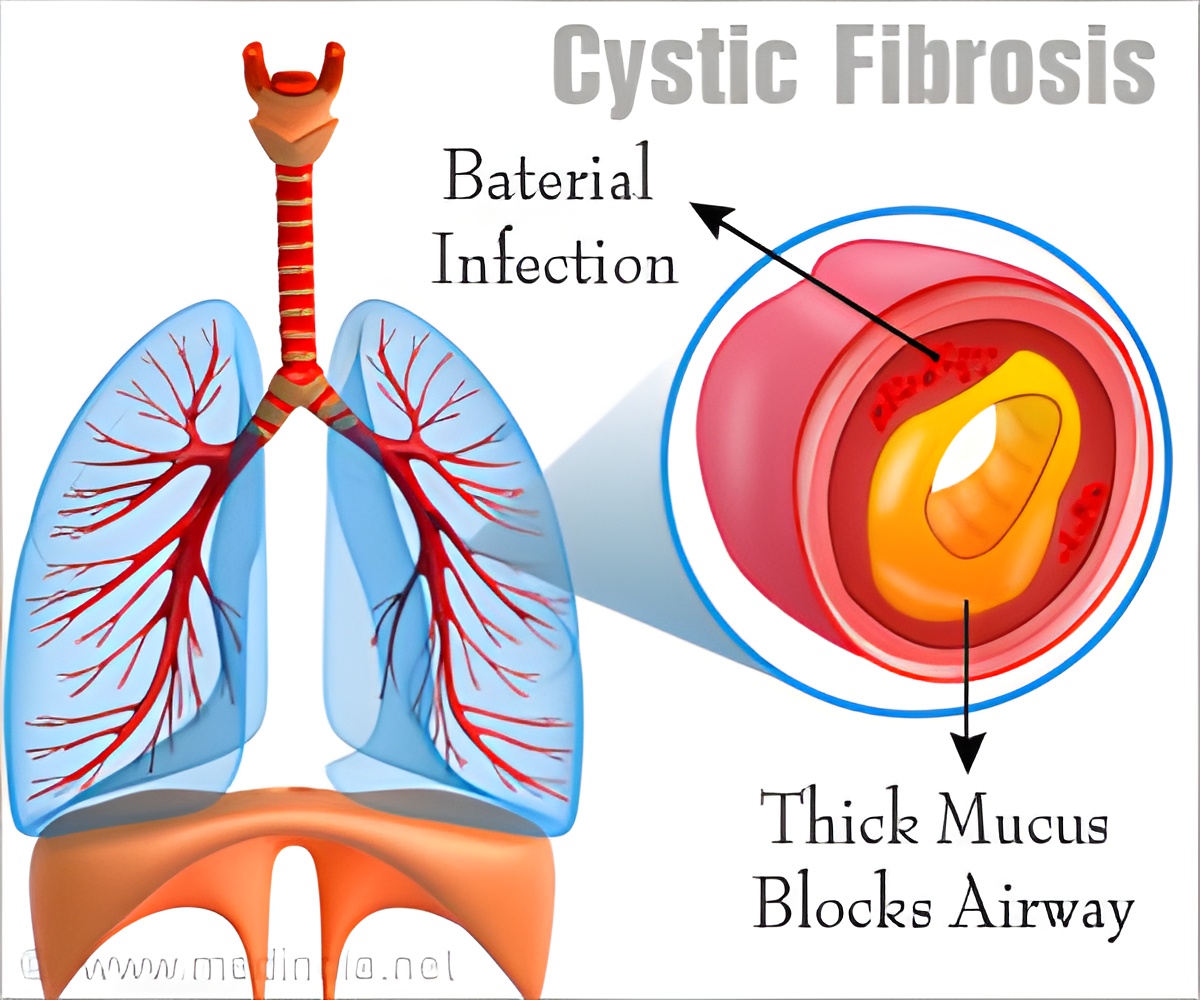
The new gene therapy has offered a significant improvement among children suffering from this respiratory disease. This treatment repairs the faulty CFTR gene by adding a healthy gene over it. Currently, this breakthrough has given new hope for developing life-saving treatments for individuals with cystic fibrosis.
The clinical trial which involved 136 patients, was carried out by Dr. Eric Alton, the co-ordinator of the UK consortium of universities and hospitals. The trial - demonstrated that the monthly dose of gene therapy over a course of a year can alter the lung function without any harmful side-effects.
Study Highlights
The £3m Phase-2 trial was conducted at the Royal Brompton Hospital in London and the Western General Hospital in Edinburgh. All patients were over the age 12 with lung function between 50-90% of normal individuals. The patients’ lung function was measured using a standard test called Forced Expiratory Volume in 1 second (FEV1). This was a Randomized Controlled Trial (RCT) to study the effectiveness of non-viral based method on cystic fibrosis.Out of 140 people with cystic fibrosis, gene therapy named as pGM169/GL67A was given to 78 patients and placebo to 62 patients. About 5ml of pGM169/GL67A containing 13.3mg of plasmid DNA and 75mg of the GL67A lipid mixture were given to the trial group and 5ml of inactive saline was given to the placebo group. They received either treatment or placebo at 28-day intervals for 12 months. Patients in both groups also received an average of three courses of oral or intravenous antibiotics during the trial.
The patients were given a nebulising spray containing fatty droplets or “liposomes” wrapped around a synthetic copy of the cystic fibrosis gene. When these liposomes were sprayed, they got absorbed by the cells on the airway linings. After getting absorbed this gene stimulated the production of healthy proteins in the cell membranes, which prevented the lungs from getting clogged with the sticky mucus.
“Patients who received the gene therapy showed a significant, if modest, benefit in tests of lung function compared with the placebo group; there were no safety concerns,” said Professor Alton.
“The phase-3 trial will focus on increasing the liposome dose and to develop a parallel treatment involving a hybrid virus that will be able to insert the healthy copy of the gene directly into the chromosomes of the affected lung cells,” said Alton.
They also believed that the gene therapy will push CF patients towards a normal life expectancy and improve their quality of life significantly. Previous studies were conducted using virus but it was shown to be ineffective. This study is a non-virus based method where only bubbles of fat were used to deliver the gene. Also, this study is a double-blind clinical trial where both the patients and scientists were unaware of who was getting the gene treatment or the placebo.
Limitations of the study
- Patients recruited in this trial were clinically stable which means they were at their optimum respiratory health during the study. Therefore, it is not known how the treatment would work in clinically unstable or very severe patient groups.
- Only 140 patients were included in this study, which was a relatively small number. To fully assess the effects of this treatment, large clinical trials are needed.
References:
1. http://www.independent.co.uk/news/science/gene-therapy-offers-hope-to-cystic-fibrosis-patients-after-successful-trials-10361634.ht2. http://www.nhs.uk/news/2015/07July/Pages/Gene-therapy-breakthrough-for-cystic-fibrosis.aspx
Source-Medindia