Novel gene therapy for wet age related macular degeneration using a virus as the gene vector appears to be safe and effective in improving vision.
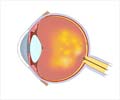
- Age-related macular degeneration (AMD) is a leading cause of blindness affecting over 1.6 million persons in the US alone.
- Current treatment involves regular injections into the eye which many patients find difficult to comply with and often end up with vision loss.
- New gene therapy has the potential to improve vision and prevent progression with a single injection.
Why Gene Therapy Might Be Better Than Current Treatments For Macular Degeneration
The team of scientists ventured to test gene therapy in patients with age-related macular degeneration, as they felt that current treatment regimens are not easy to follow. Current treatment involves administration of periodic protein injections into the eye to inactivate vascular endothelial growth factor (VEGF) responsible for the symptoms of wet AMD. The pain and discomfort associated with the injections and the need for frequent visits make the treatment difficult to follow.How Was The Therapeutic Gene Trial Conducted In Patients With AMD?
- The phase 1 clinical trial comprised 19 men and women, aged 50 years or more with advanced wet AMD.
- The scientists employed a virus similar to the common cold virus called the adeno associated virus (AAV) as the vector or carrier of the therapeutic gene.
- However, the virus was modified in the lab so that it is rendered non-pathogenic or unable to cause disease, before being incorporated with the therapeutic gene.
- The volunteers were divided into five different groups, each group being injected with increasing doses from 2X10^8 to 2X10^10 viral particles having the therapeutic gene in 0.05 mL of fluid.
- The first group was administered the lowest dose and then monitored for at least 4 weeks for evidence of toxicity before proceeding to the next group for administration of the next higher dose.
- After observing the first three groups and ensuring the absence of any dose limiting toxicity, the team administered the maximum dose to a group of ten participants and observed no serious side effects even with the maximum dose.
What Happens Following The Injection of the Therapeutic AMD Gene?
- Once injected, the harmless virus penetrates the retinal cells and deposits the therapeutic gene termed sFLT01.
- The retinal cells then virtually become factories that produce the protein encoded by the therapeutic gene in large amounts indefinitely.
- The generated protein binds to the VEGF and inactivates it making further injections unnecessary. The retinal cells will be able to produce enough protein to permanently prevent the progression of the disease.
- 4 out of the 11 patients with scope for improvement showed a dramatic response i.e. the fluid levels in their eyes dropped from severe to almost nil (as would be expected in standard treatments). 2 persons showed a partial response.
- Unfortunately, 5 patients did not show any response at all. On testing their blood, it was found that they had antibodies to the adeno virus and their immune system inactivated the virus before it could deposit the therapeutic gene into the retinal cells.
Viruses as Vectors in Gene Therapy
Viruses are employed commonly as vectors or carriers of therapeutic genes into host cells. This is because viruses have evolved mechanisms to efficiently transfer viral genes into the host cell and multiply indefinitely using the host cell machinery.Employing this property, scientists have used viruses as carriers for therapeutic gene material. The virus is modified in the lab by removing disease causing portions of its genome and replacing them with therapeutic genes to target specific cells.
Adeno associated virus is increasingly becoming a preferred choice as a vector since it is relatively non-pathogenic, can produce persistent infection in the host cell, and is capable of infecting both dividing as well as quiescent cells.
What is the Scope of this Therapeutic Gene Trial For AMD?
The research team have established the safety and efficacy of the therapeutic gene for AMD. However, they caution that it may have limitations for widespread use in the population in the future since nearly 60% of the American population has antibodies to the AAV.They, however, plan to conduct further studies in larger groups to validate their findings.
Campochiaro explains, "The numbers are small and simply show a correlation, so we don't know if serum antibodies are definitely an impediment, but more work is needed to determine this."
- Jeffrey S Heier, Saleema Kherani, Shilpa Desai, Pravin Dugel, Shalesh Kaushal, Seng H Cheng, Cheryl Delacono, Annie Purvis, Susan Richards, Annaig Le-Halpere, John Connelly, Samuel C Wadsworth, Rafael Varona, Ronald Buggage, Abraham Scaria, Prof Peter A Campochiaro, Intravitreous injection of AAV2-sFLT01 in patients with advanced neovascular age-related macular degeneration: a phase 1, open-label trial. The Lancet (2017). DOI: http://dx.doi.org/10.1016/S0140-6736(17)30979-0
Source-Medindia