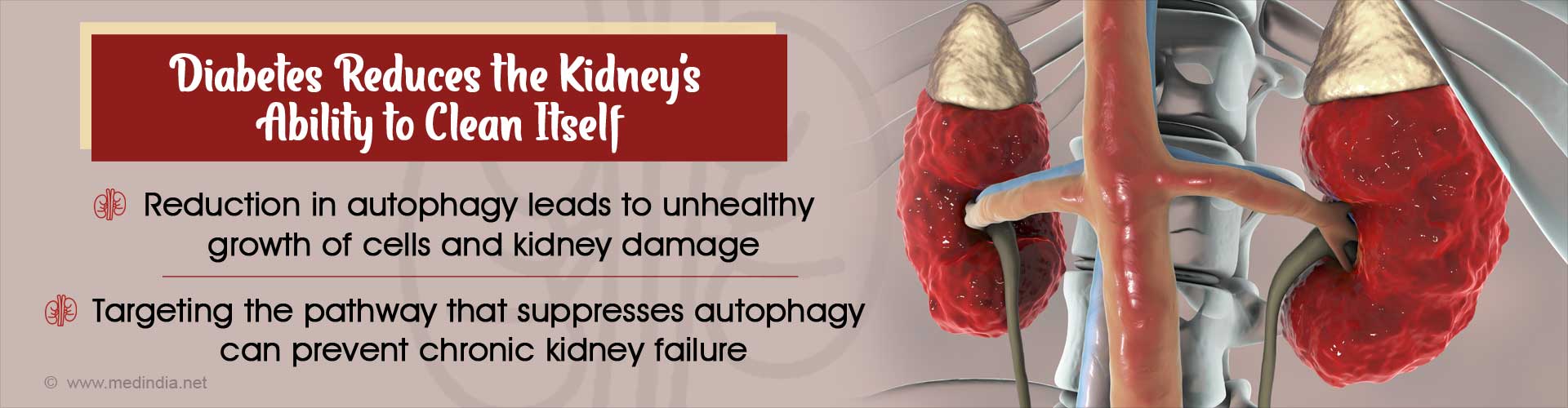
A new pathway which reduces autophagy in diabetic patients have been discovered. Reduction in autophagy dramatically reduces the kidney’s ability to clean itself.
- Autophagy in diabetes initially increases and then reduces due to the activation of a pathway
- Tumor suppressor gene p53 activates miRNA miR-214 and inhibits autophagy gene ULK1
- Inhibition of autophagy gene results in unhealthy overgrowth of cells leading to hypertrophy and kidney disease
- Targeting miR-214 can potentially prevent kidney failure in diabetes as it is in the way of autophagy but does not have an obvious role in kidney function
Read More..
One of the major complications of diabetes is diabetic kidney disease. Diabetes is the leading cause of chronic kidney disease and kidney failure.
Autophagy
Autophagy means ‘self-eating’. It is the body’s way of cleanup of debris, misfolded proteins and mitochondria.
The damaged cellular components are packed into a double-membraned sack and get destroyed with the help of enzymes.
Autophagy generally increases in diseases like diabetes. However, scientists have found a new pathway that causes autophagy dysfunction in chronic kidney disease like diabetes.
Many scientists who had studied diabetes and kidney disease were giving conflicting reports on the level of autophagy. Few reported that there was high autophagy, whereas few reported that it went down.
Study Details and Findings
The researchers used levels of the protein LC3 as a reliable biomarker. LC3 is a protein that is found in the membranes of the sacks that are involved in the elimination of the damaged cell organelles.
High activity of p53 and miR-214 and low activity of ULK1 and activity was observed in both animals and humans over a short period.
In the mouse model with type 1 diabetes, there was no difference in the biomarker when compared to healthy controls at 9 weeks.
Two weeks later, the biomarker reduced in diabetic mice and the level significantly reduced at 11 weeks. By 20 weeks there was protein in the urine and kidney failure.
The diabetic mice were smaller but had larger kidneys due to hypertrophy and signs of renal tubule damage.
The researchers found that a tumor suppressor gene called p53 activates a microRNA called miR-214, which suppresses the autophagy gene ULK1.
Once the microRNA is activated, there is an overgrowth of cells and the kidney, which leads to hypertrophy, scarring, inflammation and protein spilling into the urine. This is a classic sign of kidney failure.
The gene ULK1 was low in both diabetes models and kidney biopsies from diabetic patients.
When miR-214 was knocked out in the kidney’s filtering units, ULK1 expression and autophagy activity did not decrease. Blocking p53 did not increase the level of miR-214 levels, and the downstream benefits of blocking its action were essentially the same.
The researchers also compared human samples from healthy people and diabetic patients. They found that there were no signs of p53 in none of the normal samples. Whereas, around 15-20 kidney biopsies of diabetic patients had signs of p53.
Similar findings were observed about miR-214. The healthy kidneys also had a high level of ULK1, and diabetes biopsies had low levels of the gene.
miR-214 can be potentially used as an intervention target, and kidney failure progress can be slowed down and prevented.
The researchers are not certain if impaired autophagy completely explains the unhealthy growth of cells. However, they are confident that autophagy contributes to unhealthy growth.
Source-Medindia