Mepolizumab has been approved by the US FDA to treat eosinophilic granulomatosis with polyangiitis, providing patients with the autoimmune disorder hope.
Highlights:
- Mepolizumab is a monoclonal antibody used in eosinophilic asthma that has now been approved for eosinophilic granulomatosis with polyangiitis
- It appears to increase the total duration of remission as well as the number of patients who achieve remission, thereby reducing the required dose of corticosteroid
- It may, however, not be effective in all individuals with the disease.
- The treatment with mepolizumab was associated with a longer total disease-free duration. The percentage of patients who were free from the disease within 24 weeks was higher (28%) in the mepolizumab group as compared to those who received placebo (3%).
- A higher percentage of individuals on mepolizumab achieved remission at week 36 and week 48 of the trial as compared to placebo.
- Almost half the patients (44%) in the mepolizumab group and 81% in the placebo group did not achieve remission. Thus, though the above numbers depict an advantage of mepolizumab over placebo, it is possible that a large number of individuals treated with the drug may not respond to it.
- Treatment with mepolizumab allowed the reduction of the corticosteroid dose. Only around 7% patients on placebo could reduce their prednisone or prednisolone dose to 4 mg or less per day between 48 and 52 weeks of treatment; in contrast, this was achieved by 44% patients in the mepolizumab group. A lower dose of corticosteroid translates into lesser side effects caused by its long-term use.
- The adverse effects reported in the study included headache, upper respiratory tract infection, injection-site reaction and worsening asthma.
About Mepolizumab
Mepolizumab is an anti-interleukin 5 monoclonal antibody. Interleukin 5 controls the proliferation, maturation and differentiation of white blood cells called eosinophils, which play an important role in allergic reactions. Mepolizumab reduces the number of eosinophils in the patient, which explains its effectiveness in eosinophilic asthma where it is used in a dose of 100 mg. The approved dose for eosinophilic granulomatosis with polyangiitis is a subcutaneous injection of 300 mg once every four weeks into the upper arm, thigh or abdomen.About Eosinophilic Granulomatosis with Polyangiitis
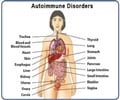
Eosinophilic granulomatosis with polyangiitis (EGPA), previously called Churg-Strauss syndrome, is a rare autoimmune disease that results in inflammation of small-to-medium blood vessels, adult-onset asthma and high levels of eosinophils in the blood. It can affect the nasal sinuses, skin, heart, digestive tract and the nervous system. Frequent relapses can lead to organ damage. It is usually treated with corticosteroids, sometimes with additional immunosuppressant drugs.References:
- FDA approves first drug for Eosinophilic Granulomatosis with Polyangiitis, a rare disease formerly known as the Churg-Strauss Syndrome - (https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm588594.htm)
- Wechsler ME et al. Mepolizumab or Placebo for Eosinophilic Granulomatosis with Polyangiitis. N Engl J Med 2017; 376:1921-193. DOI: 10.1056/NEJMoa1702079
Source-Medindia