Clinical trials are evaluating two new drugs, tofacitinib and guselkumab, for the treatment of psoriatic arthritis. The results appear promising.
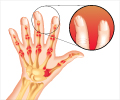
- Psoriatic arthritis is treated with medications which include DMARDs, anti-TNFα agents, NSAIDs for pain relief and corticosteroids as adjuvant treatment
- Physical therapy also plays an important role in the treatment of psoriatic arthritis
- Two new drugs, tofacitinib and guselkumab, are being evaluated in clinical trials for the treatment of psoriatic arthritis
The two studies evaluated the use of the drugs, tofacitinib and guselkumab, for the treatment of psoriatic arthritis. While the study evaluating tofacitinib was a phase 3 clinical trial and evaluated the drug in a large number of patients, guselkumab was a phase 2 clinical trial, which evaluated the drug in a smaller number of patients.
Tofacitinib, an oral medication, had earlier received approval for the treatment of rheumatoid arthritis. It is an anti-inflammatory drug that belongs to a group called janus kinase inhibitors.
The study evaluated tofacitinib in 422 patients with active psoriatic arthritis. These patients:
- Were suffering from psoriatic arthritis for a minimum period of 6 months
- Did not respond well to at least one conventional synthetic DMARD
- Did not previously receive anti-TNFα treatment
- Were mostly Caucasians, and about half of them were female
The researchers found that:
- Tofacitinib was more effective than placebo in the treatment of psoriatic arthritis. The benefits of the treatment were noted, as early as 2 weeks, and continued for a year.
- In around 90% individuals, the disease did not progress in 1 year, as demonstrated through radiological tests.
- The side effects were similar to those noted in previous studies and included upper respiratory tract infection and headache.
The second study evaluated the use of guselkumab, a monoclonal antibody, in 149 patients with psoriatic arthritis. Monoclonal antibodies are proteins produced in the laboratory that show a specific effect against a particular disease. The patients
- Also had 3% of their body surface area affected with psoriasis
- Had received the usual prescribed treatments, which may have included anti-TNFα agents.
Guselkumab was administered at a dose of 100 mg through a subcutaneous injection, which is administered just under the skin. The injection was administered at the start of the study, at week 4, and then every 8 weekly till week 44. The researchers found that guselkumab, as compared to placebo:
- Improved outcomes in psoriatic arthritis at week 24 of treatment. Some patients experienced the benefits as early as 4 weeks of treatment
- Improved enthesitis. Enthesitis refers to inflammation of the sites where the tendons or ligaments are attached to the bones. It stimulates bone formation at these sites, and can be painful
- Improved dactylitis, a condition where the fingers and toes are affected
- Reduced the disease activity and improved quality of life
- Caused side effects that were expected and included infection. One patient had a knee injury and another had a heart attack
- Mease PJ et al. Efficacy and Safety of Tofacitinib, an oral Janus Kinase Inhibitor, or Adalimumab in Patients with Active Psoriatic Arthritis and an Inadequate Response to Conventional Synthetic Disease-Modifying Antirheumatic Drugs (CSDMARDS): A Randomized, Placebo-controlled, Phase 3 Trial. Abstract No. OP0216 EULAR 2017. DOI: 10.1136/annrheumdis-2017-eular.1416
- Deodhar A et al. Efficacy and Safety Results of Guselkumab, an anti-IL23 Monoclonal Antibody, in Patients with Active Psoriatic Arthritis over 24 weeks: A Phase 2A, Randomized, Double-Blind, Placebo-Controlled Study. Abstract No. OP0218 EULAR 2017. DOI: 10.1136/annrheumdis-2017-eular.1164
Source-Medindia