Surgical implants with tubular braided sleeves around biodegradable cores that accommodate tissue growth could reduce the need for repeated implant replacement in children.
Highlights:
- Surgical implants have now been designed that accommodate tissue growth
- The devices consist of tubular braided sleeves around biodegradable cores
- Further improvements in the design could help to fine tune the device for specific clinical indications
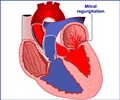
Artificial implants are used surgically to correct structural defects. Annuloplasty ring implants are often used to repair defects in the heart valves. However, since the implants are of fixed size, they do not accommodate for the growth in children. Similarly, for limb deformities in children with limb length discrepancy and angular limb deformities, fixed size implants are currently used, which require another surgery to remove the implants at the right time to prevent growth arrest. Unfortunately, following removal, the child is at risk for rebound growth. A similar problem exists for the treatment of scoliosis.
The scientists tested the device in the tibia of rats. On examination with micro-CT, the core thinned with time and the device length increased. The device elongation was confirmed on removal. The device also supported a more physiological growth of the tibia, as compared to rats who received fixed devices.
The device was also tested as tricuspid heart valves in growing piglets. In these cases, the polymer erosion and ring diameter decrease were variable in different segments. This could have been due to the collagen growth on the valve, which could have slowed the degradation. However, the device supported valve growth and valve stenosis, regurgitation or peri-prosthetic leak did not occur.
Further changes in the device through research could help to fine tune the device for specific requirements. Modified versions of the device could be useful in conditions like esophageal and intestinal atresia, and mandibular condylar hyperplasia (a condition affecting the lower jaw bone). The devices can be modified to match the predicted growth rate of the child. The use of several layers of cores of different materials that degrade at different rates could be used, thus allowing for the lengthening of the braid at different rates at different times. The core could be made of material that gets degraded on exposure to infrared light applied externally. Thus, it would be possible to time the degradation.
Reference:
- Feins EN et al. A growth-accommodating implant for paediatric applications. Nature Biomedical Engineering 1, 818–825 (2017) doi:10.1038/s41551-017-0142-5
Source-Medindia