Novel complement inhibitor therapy developed that reduces nerve cell damage and brain tissue inflammation following stroke with improved patient outcomes.
- Scientists develop novel “complement inhibitor” therapy that reduces brain tissue inflammation and nerve cell damage following stroke with better recovery of neurological deficits.
- Currently tissue plasminogen activator (tPA), a so-called 'clot buster' drug, is the only pharmacological stroke intervention available has certain limitations.
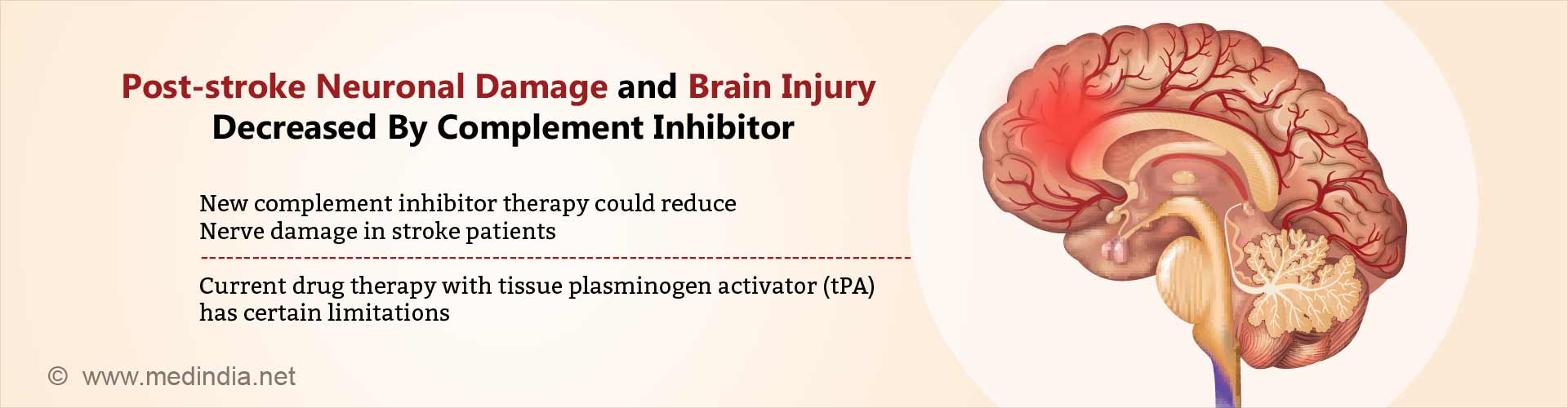
- Ischemic stroke due to clot is one of the major causes of mortality and morbidity worldwide, especially in the elderly.
Aim of New Complement Inhibitor Therapy
The team believe that the inhibition of complement will limit neuronal damage to the core area of stroke while preventing the extension of damage to the adjacent areas, thereby increasing the area of brain involved. "There's an ischemic core where the greatest oxygen deprivation occurs during an ischemic stroke. Neurons in that area are irreparably damaged and die. But damaged neurons outside the stroke core can be salvaged," explains principal investigator and senior author Stephen Tomlinson, Ph.D., professor and vice chair for research and faculty development in MUSC's Department of Microbiology & Immunology."Unfortunately, complement becomes activated and signals that these damaged neurons should be cleared from the brain before they get a chance to recover."
Analyzing Effect of Complement in Stroke
The study team did a series of experiments to outline the role of complement in stroke since it contributes to neuro-degenerative as well as neuro-regenerative processes. During these tests, they found that microglial phagocytosis (ingestion by microglia) of neurons, a previously described phenomenon also occurs after stroke."We were seeing neuronal material inside the microglial cells and, at first, we thought it must be some sort of artifact. It was a surprise," says Alawieh "So, we repeated the experiment several times to be sure that phagocytosis of live neurons was really what was happening. We didn't previously know that occurred in the perilesional area after stroke."
- The team found that live but stressed neurons around the stroke area display danger-associated molecular patterns (DAMPs; also called neoepitopes) that stimulate complement C3d deposition on the outer cell membrane. The C3d marks the neuron for rapid elimination by microglia cells.
- This 'quick response' mechanism helps explain the rapid loss of neurons observed in the perilesional area.
- Even more importantly, this mechanism also promotes a pro-inflammatory environment that drives significant chronic inflammation and tissue damage after the stroke.
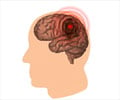
Why Brain Tissue Adjacent to Stroke Area Becomes Damaged
The cells injured and destroyed by stroke release harmful chemicals into the adjacent area and these neurons temporarily go into a “shut-down mode” as a protective response. The patient’s immune system including complement mistakenly recognizes these non-functioning cells as damaged cells that have to be cleared and thus these normal cells are also eliminated (increasing the area of injury).Therefore, in stroke, complement becomes pathological and inappropriately marks live neurons for elimination.
Testing Complement Inhibitor Therapy in Preclinical Model – Study Details
- To inhibit the above processes, the team created a novel therapeutic agent to inhibit complement activation locally in areas where nerve cells express DAMPs (or neoepitopes).
- They joined an antibody fragment that recognizes a post-ischemic neoepitope (the vehicle) to a complement inhibitor dubbed B4Crry- and tested it in a murine stroke model.
- They B4Crry was found to bind to hypoxic (oxygen-deprived) cells and inhibit complement activation but did not bind to normally oxygenated cells.
- The B4Crry had a short circulatory half-life but a prolonged (35-hour) tissue half-life, ensuring that complement inhibition would be limited to affected brain tissue and not affect systemic serum complement activation.
- Animals treated with B4Crry showed reduced C3d deposition in the post-stroke brain, lesser neurological deficits and a lesser infarct volume 24 hours post-stroke in comparison to control animals.
- Over a 15-day recovery period, B4Crry-treated animals had fewer neurological deficits and smaller lesions as well as better recovery of initial deficits compared to control animals.
- Overall, the B4Crry-treated group showed quicker learning curves, better learned memory retention, and a four-fold increase in cortical and hippocampal neuroblasts compared to controls animals.
- Importantly, B4Crry was found to be effective in male, female, adult and aged animals, and effects were present over a 30-day period and in animals treated as late as 24 hours post-stroke.
Potential Merits of the Novel B4Crry Agent
- B4Crry shows a longer treatment window than for tissue plasminogen activator (tPA), a so-called 'clot buster' drug, currently the only pharmacological therapy available.
- Also, unlike tPA, B4Crry, could in theory be administered to persons who are at risk for bleeding, a finding that needs to be validated in human clinical trials.
- B4Crry, did not increase infection risk, since systemic complement inhibition is known to increase risk of infections following stroke, thus offering a critical advantage.
Scope of Study and Future Plans
The team has already investigated the role of this approach in other conditions, such as cardiovascular diseaseThey plan to further study complement inhibition treatment in Traumatic Brain Injury (TBI) to understand how complement-dependent mechanisms impact the prognosis of TBI
They have demonstrated that the B4 epitope is expressed on other damaged human tissues as well and plan to take forward B4Crry therapy in clinical trials with human patients in the near future.
References:
- Ali Alawieh, E. Farris Langley and Stephen Tomlinson, "Targeted complement inhibition salvages stressed neurons and inhibits neuroinflammation after stroke in mice", Science Translational Medicine (2018) DOI: 10.1126/scitranslmed.aao6459
Source-Medindia