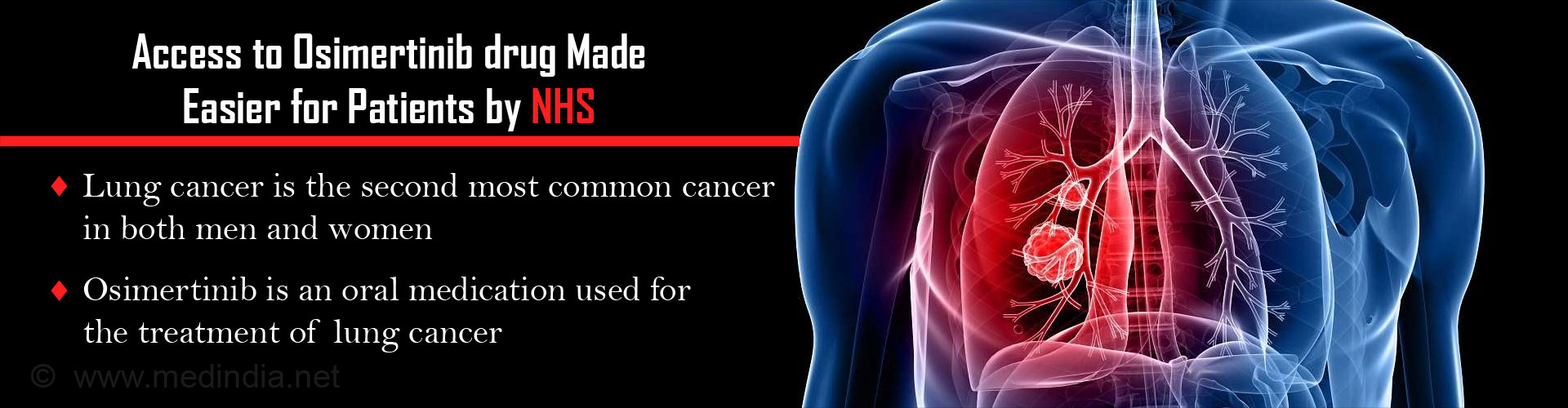
The availability of the anti-cancer drug osimertinib through the NHS will make it more accessible to EGFR T790M-positive lung cancer patients in the UK.
Highlights
- Mutations in EGRF (epidermal growth factor receptor) predispose to non-small cell lung cancer (NSCLC).
- The cancer can therefore be treated with drugs called EGFR tyrosine kinase inhibitors (EGRF-TKI) which include erlotinib, gefitinib, and afatinib.
- Patients on EGRF-TKIs soon develop resistance to treatment. Newer drugs like osimertinib may benefit these patients.
Exposure to cigarette smoke, radiation or occupational pollutants can predispose to NSCLC. It is often diagnosed late and has a poor prognosis. Mutations in EGRF (epidermal growth factor receptor) cause the lung cells to grow into cancer. Therefore, a group of drugs called EGFR tyrosine kinase inhibitors (EGRF-TKI) are used to treat it. Drugs in this group are erlotinib, gefitinib, and afatinib. Unfortunately, EGRF-TKIs become ineffective after being used for a few months following the development of resistance due to mutations like the T790M mutation in the EGRF gene. The newer EGRF-TKI osimertinib also targets the T790M mutation, and is therefore used in the treatment of resistant cancers. Osimertinib provides the last chance of hope for these cancer patients.
Lung Cancer - Facts and Statistics
- Lung cancer is the second most common cancer in both men and women
- About 224,390 new cases of lung cancer in 2016
- 14% of new cancers diagnosed are lung cancers
- Lung cancer accounts for about 1 out of 4 cancer deaths
- It mainly occurs in older people
- Smoking is the primary cause of lung cancer
Common side effects include diarrhea, skin rash, dry skin, and nail changes. More serious adverse effects include lung and heart disease. Osimertinib should not be used in pregnancy.
Though short-term results of studies indicate that this new drug is advantageous, sometimes even bringing an apparent cure from cancer, its long-term effectiveness will be determined through ongoing clinical studies.
- NICE Recommends New Drug Osimertinib for Hundreds of People with Lung Cancer - (https://www.nice.org.uk/news/article/nice-recommends-new-drug-osimertinib-for-hundreds-of-people-with-lung-cancer)
- Non-Small Cell Lung Cancer Treatment - (https://www.cancer.gov/types/lung/hp/non-small-cell-lung-treatment-pdq)
- Highlights of Prescribing Information - (http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/208065s000lbl.pdf)
- Key Statistics for Lung Cancer - (http://www.cancer.org/cancer/lungcancer-non-smallcell/detailedguide/non-small-cell-lung-cancer-key-statistics)