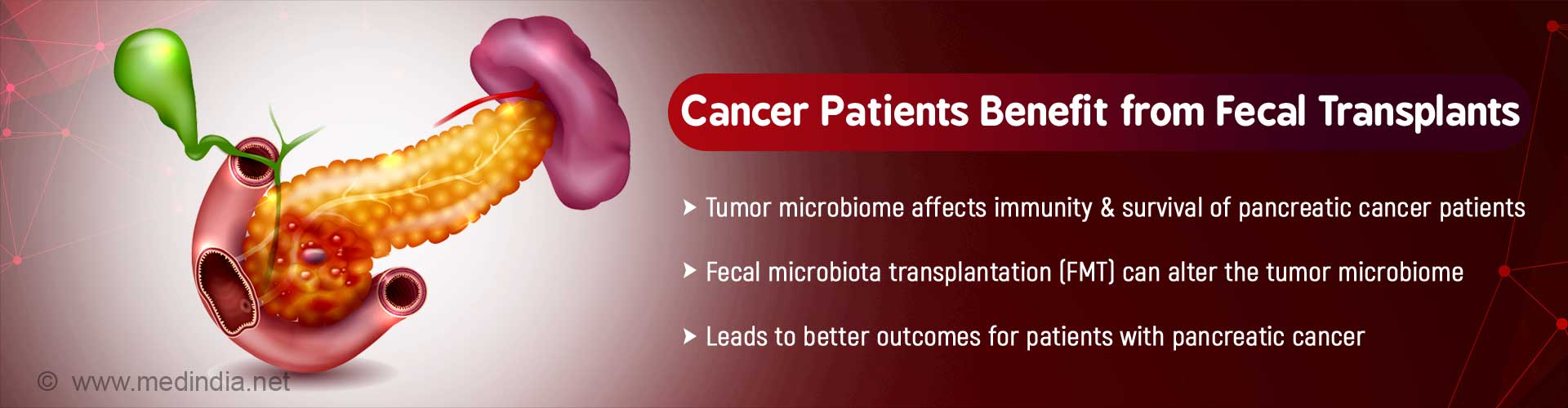
Bacteria on tumors can affect immune response and survival of patients with pancreatic cancer. The tumor microbiome can be altered by fecal microbiota transplantation (FMT), which leads to better outcomes.
- Bacteria on tumors can influence the immune response and survival of pancreatic cancer patients
- The tumor microbiome can be altered by fecal microbiota transplantation (FMT)
- This can significantly improve the outcome for patients with pancreatic cancer
The study, published in Cell, was led by Dr. Florencia McAllister, MD, who holds joint appointments as Assistant Professor in the Department of Clinical Cancer Prevention and Department of Gastrointestinal Medical Oncology at the University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Read More..
What are the Problems Associated with Pancreatic Cancer?
The most common type of pancreatic cancer is pancreatic ductal adenocarcinoma. This type of pancreatic cancer has problems both when it is diagnosed at a late-stage or at an early-stage. On the one hand, when the cancer is diagnosed at a late-stage, only 9 percent of patients survive till five years. On the other hand, when the cancer is detected at an early stage and surgically treated, there is a high recurrence rate, as a result of which the median duration of survival of the patients is only 24-30 months. Moreover, no genomic markers have been identified that can explain the reason for the long-term survival of some of the patients.What is the Role of Tumor Microbiome on Pancreatic Cancer Survival?
Gut microbiome plays an important role in modulation of cancer immunotherapy. However, it is not known whether the bacterial flora present on tumors – the tumor microbiome – also plays a role in cancer.To answer this question, the research team analyzed the DNA (deoxyribonucleic acid) of bacteria present on tumors from long-term pancreatic cancer survivors and compared it with that from short-term survivors. The study included two independent cohorts – one from MD Anderson Cancer Center and the other from Johns Hopkins University.
In the MD Anderson cohort, the median long-term survival was 10 years (22 patients) and the median short-term survival was 1.6 years (21 patients). In the case of the Johns Hopkins cohort used for validation purposes, 15 patients survived longer than 10 years, while 10 survived less than five years.
Sequencing the 16S rRNA (ribosomal ribonucleic acid) gene, clearly showed that the long-term pancreatic cancer survivors had a greater bacterial diversity on the surface of the tumors, compared to short-term survivors. Based on the bacterial diversity, it was found that a high bacterial diversity was linked to longer survival (median survival of 9.66 years), while low bacterial diversity was linked to shorter survival (median survival of 1.66 years).
These findings, based on bacterial diversity, were independent of other factors such as prior cancer therapy, exposure to antibiotics, and body mass index (BMI). This makes bacterial diversity a good predictor of survival in the case of patients undergoing pancreatic cancer surgery. Moreover, it also acts as an excellent biomarker for monitoring the progression of pancreatic cancer.
Another major finding was that the preponderance of particular bacterial species on the tumors was associated with the duration of survival. For example, long-term survivors had larger numbers of bacteria belonging to the genus Pseudoxanthomonas, Saccharopolyspora, and Streptomyces, as well as the bacterium Bacillus clausii. Presence of these species of bacteria predicted a better outcome for patients in both the MD Anderson and Johns Hopkins cohorts.
What is the Link between Tumor Microbiome and the Immune System?
Tumor microbiome is associated with immunity. Immunohistochemical techniques revealed that in the tumors of long-term pancreatic cancer survivors of both the MD Anderson and Johns Hopkins cohorts, there were greater numbers of T-cells, particularly CD8+ T-cells, which are responsible for cell-mediated immunity. These observations corroborated with previous studies which found that long-term survivors exhibited more vigorous immune responses.The research team also found that immune cell infiltration was strongly correlated with bacterial diversity of the tumors. Moreover, immune cell infiltration and T-cell activation were strongly linked to the abundance of the three types of bacteria detected on the surface of tumors of long-term pancreatic cancer survivors.
The establishment of this link between the tumor microbiome and the immune system encouraged the researchers to look for a way to alter the tumor microbiome.
Can Tumor Microbiome be Altered by Gut Microbiome?
The tumor microbiome cannot be altered directly. However, it was hypothesized that the gut microbiome could possibly alter the tumor microbiome, if there was a cross-talk between the two microbiomes.Bacteria in the gut, in the tumors and in adjacent tissues were compared in 3 patients undergoing surgery. It was found that the gut microbiome represented 25 percent of the tumor microbiome. However, these gut bacteria were absent in the normal, adjacent tissues. This indicated that there was indeed a link between the gut microbiome and tumor microbiome and that the gut bacteria were capable of colonizing pancreatic tumors.
Fecal microbiota transplantation (FMT) from patients with advanced pancreatic cancer into mice revealed that the gut microbiome from the patients represented around 5 percent of the resultant tumor microbiome in mice. Moreover, FMT altered 70 percent of the total tumor microbiome. This finding proved that FMT was capable of completely altering the bacterial population of the tumor microbiome.
Is Fecal Microbiota Transplantation (FMT) Dependent on the Immune System?
FMT was performed in mice from patients with advanced pancreatic cancer, long-term survivors, and healthy controls. Five weeks after the fecal transplantation, it was observed that the mice which received FMT from advanced cancer patients had much larger tumors than those which received FMT from long-term survivors (70% smaller tumor size) or healthy controls (50% smaller tumor size).Immunological studies revealed that the mice which received FMT from long-term survivors had higher levels of activated CD8+ T-cells compared to the other two groups. The mice that received FMT from patients with advanced pancreatic cancer had higher levels of regulatory T-cells (Tregs) and myeloid-derived suppressor cells (MDSC), both of which are capable of suppressing the immune response.
In order to evaluate whether the effect of FMT was dependent on the immune system, T-cells were depleted in mice that received fecal transplants from long-term survivors. This led to complete blocking of anti-tumor activity of the fecal transplants. This clearly showed that the effect of FMT is dependent on the immune system.
Concluding Remarks
McAllister concluded:“Results of the FMT experiments represent a significant therapeutic opportunity to improve pancreatic cancer treatment by altering the tumor immune microenvironment.” She added: “There is promise here but we have a lot of work ahead.”Funding Source
The research was funded by multiple organizations, foundations, institutes, and award grants. Some of these include the American Gastroenterological Association Research Foundation, MD Anderson’s Moon Shots ProgramTM, MD Anderson Cancer Center, Stanford University-Emerson Collective Cancer Research Fund, Stand Up To Cancer - Lustgarten Foundation, the National Cancer Institute of the National Institutes of Health, the Pancreatic Cancer Action Network-AACR Career Development Award, and the Paul Calabresi Career Development Award for Clinical Oncology (PCACO) K12.Reference:
- Pancreatic cancer - (https://www.mdanderson.org/newsroom/bacteria-on-tumors-influences-immune-response-and-survival-of-pa.h00-159305412.html)
Source-Medindia