Results from a clinical trial, FOURIER, suggest that PCSK9 inhibitor, evolocumab is safe and efficient in treating peripheral artery disease.
- Results of clinical trial FOURIER, show that addition of PCSK9 inhibitor evolocumab to statin therapy reduces cardiovascular risk.
- Evolocumab works by drastically reducing LDL cholesterol, which is a risk factor for cardiovascular diseases.
- Evolocumab efficacy is especially beneficial to people affected by peripheral artery diseases as they are at high risk for heart-related diseases.
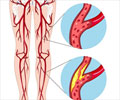
Peripheral Artery Disease (PAD)
Peripheral Artery Disease is a circulatory problem where the blood circulation to the lower limbs is limited due to narrow arteries. Patients with PAD are at heightened risk of major adverse cardiovascular events including myocardial infarction, stroke, and cardiovascular death. PAD is also majorly associated with major adverse limb events including acute limb ischemia and major amputation. It is usually caused due to fatty deposits in the arteries called atherosclerosis.Treatment
Despite the best treatments using high-intensity statins, the risk of cardiovascular and limb events remains high. While low-density lipoprotein (LDL) cholesterol, a modifiable risk factor for cardiovascular disease, has been studied in association with atherosclerosis, very few studies show its efficiency in treating PAD patients.Evolocumab
Evolocumab is a member of a new class of cholesterol-lowering drugs known as PCSK9 inhibitors. This group of drugs have emerged as an effective treatment in lowering LDL to an extent that is not possible by statin therapy alone. Evolocumab reduces LDL cholesterol levels by approximately 60%.FOURIER Clinical Trial
FOURIER was a very large trial involving 27,564 patients with either atherosclerosis, PAD, myocardial infarctions, stoke and other conditions in 49 countries, who were on statin therapy. The aim of the trial was to determine if the addition of evolocumab to standard statin therapy reduced the risk of adverse cardiovascular events. This was a randomized trial where some received evolocumab while others received the placebo. Patients were observed for approximately 2.5 years for cardiovascular and limb events. After analysis of the FOURIER data, it was found that evolocumab reduced the risk of cardiovascular disease in patients with and without PAD. As patients with PAD are at a higher risk of a heart attack and stroke, these patients had a higher absolute risk reduction of 3.5% while patients without PAD had a 1.6% reduced risk of a cardiovascular event. The risk of major adverse limb events was reduced to half in patients who received evolocumab compared to PAD patients who received the placebo. In PAD patients who had no history of a heart attack or stroke, evolocumab reduced risk of a cardiovascular or adverse limb event by 6 percent over 2.5 years. The usage of evolocumab was not associated with any concerning safety issues.References:
- Bonaca, M. et al. Low-Density Lipoprotein Cholesterol Lowering With Evolocumab and Outcomes in Patients With Peripheral Artery Disease: Insights From the FOURIER Trial (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk). CirculationCIRCULATIONAHA.117.032235 (2017). doi:10.1161/circulationaha.117.032235