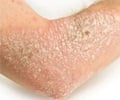
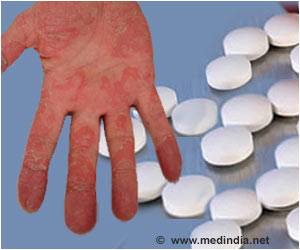
Brodalumab a monoclonal antibody designed to block the function of IL-17 has shown 100% decrease in symptoms among people with moderate to severe psoriasis.
The severity of psoriasis is typically graded as mild or moderate to severe depending on the extent of skin involvement and the impact the disease has on the individual’s health-related quality of life. There may be times when the symptoms of psoriasis get better while on other occasions they might worsen. There is no permanent cure for psoriasis. But, treatments may offer significant relief.
The exact cause of psoriasis is not known. It is believed to be associated with environmental and genetic factors that lead to disturbances in the immune responses tothe skin. The immune system signaling protein interleukin 17 (IL-17) that controls cells and activates inflammation, is thought to be a potent driver of psoriasis.
In a normal person, when there is a cut or a scrape, IL-17 signals the body's immune system to send cells to the surface to fight infection and heal the wound. In people with psoriasis, these immune signals can be faulty, and the immune system gets mobilized for an unknown reason. People with psoriasis lesions have 30 times more IL-17 than people without lesions.
In a bid to offer better treatment options for psoriasis, clinical trials with drugs that block the function of IL-17 are being conducted. Results of a multicenter clinical trial led by Mount Sinai researchers has revealed that an experimental and biologic treatment, called brodalumab, has achieved 100% decrease in psoriasis symptoms in twice as many patients as a second, commonly used treatment.
Brodalumab is a monoclonal antibody that was designed to block the function of IL-17. Early clinical studies have suggested brodalumab to be efficacious in the treatment of psoriasis.
Ustekinumab is already approved by the United States Food and Drug Administration (FDA) and is widely used for the treatment of psoriasis. It blocks the related inflammatory signalling chemicals or cytokines (IL-12 and IL-23).
At week 12, the study subjects who received brodalumab were randomly put on a brodalumab maintenance dose of 210 mg every 2 weeks or brodalumab 140 mg every 2 weeks, every 4 weeks, or every 8 weeks. Patients who were given ustekinumab continued with the same every 12 weeks. Study participants who received placebo, were then assigned to receive 210 mg of brodalumab every 2 weeks.
The treatment efficacy was measured as the degree of reduction in the Psoriasis Area Severity Index or PASI, which assesses redness, scaling and thickening of the psoriatic skin lesions and the extent of the disease. A PASI 100 refers to a 100% reduction in symptoms.
After 12 weeks, 44% of patients who were randomized to receive 210 mg dosage of brodalumab every two weeks, had achieved PASI 100, compared with 22% of patients treated with ustekinumab.
In the second study, 37% of patients who were randomized to receive 210 mg brodalumab every two weeks achieved PASI 100, as compared with 19% of patients treated with ustekinumab. With 140 mg of brodalumab, the PASI 100 response rates were 26%.
86% of patients reportedly achieved PASI 75 with 210 mg brodalumab, and 67% patients achieved PSAI 75 with 140 mg brodalumab.
The research team observed that episodes of low white blood cell count were higher with brodalumab and with ustekinumab as compared to that of placebo. Mild or moderate fungal infections were more commonly reported with brodalumab than with ustekinumab or placebo. The overall frequencies of the most common adverse events such as upper respiratory tract infection, headache and joint pain were similar between the treatment and placebo group.
The researchers concluded that treatment with brodalumab resulted in significant clinical improvements in patients presenting with moderate to severe psoriasis. The results of the current studies will be relevant when the FDA considers the application of brodalumab for psoriasis treatment.
The research has been published online in the New England Journal of Medicine.
References:
1. http://www.nejm.org/doi/full/10.1056/NEJMoa15038242. http://www.eurekalert.org/pub_releases/2015-09/tmsh-ate092515.php
Source-Medindia